Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study-A Randomized Clinical Trial
- PMID: 27678080
- PMCID: PMC5115200
- DOI: 10.1111/jvim.14586
Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study-A Randomized Clinical Trial
Abstract
Background: Pimobendan is effective in treatment of dogs with congestive heart failure (CHF) secondary to myxomatous mitral valve disease (MMVD). Its effect on dogs before the onset of CHF is unknown.
Hypothesis/objectives: Administration of pimobendan (0.4-0.6 mg/kg/d in divided doses) to dogs with increased heart size secondary to preclinical MMVD, not receiving other cardiovascular medications, will delay the onset of signs of CHF, cardiac-related death, or euthanasia.
Animals: 360 client-owned dogs with MMVD with left atrial-to-aortic ratio ≥1.6, normalized left ventricular internal diameter in diastole ≥1.7, and vertebral heart sum >10.5.
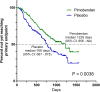
Methods: Prospective, randomized, placebo-controlled, blinded, multicenter clinical trial. Primary outcome variable was time to a composite of the onset of CHF, cardiac-related death, or euthanasia.
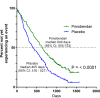
Results: Median time to primary endpoint was 1228 days (95% CI: 856-NA) in the pimobendan group and 766 days (95% CI: 667-875) in the placebo group (P = .0038). Hazard ratio for the pimobendan group was 0.64 (95% CI: 0.47-0.87) compared with the placebo group. The benefit persisted after adjustment for other variables. Adverse events were not different between treatment groups. Dogs in the pimobendan group lived longer (median survival time was 1059 days (95% CI: 952-NA) in the pimobendan group and 902 days (95% CI: 747-1061) in the placebo group) (P = .012).
Conclusions and clinical importance: Administration of pimobendan to dogs with MMVD and echocardiographic and radiographic evidence of cardiomegaly results in prolongation of preclinical period and is safe and well tolerated. Prolongation of preclinical period by approximately 15 months represents substantial clinical benefit.
Keywords: Asymptomatic; Echocardiography; Endocardiosis; Heart failure; Mitral regurgitation; Prevention; Treatment; Vertebral heart sum.
Copyright © 2016 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals, Inc. on behalf of the American College of Veterinary Internal Medicine.
Figures

References
-
- Buchanan JW. Prevalence of cardiovascular disorders In: Fox PR, Sisson D, Moise NS, eds. Textbook of Canine and Feline Cardiology. Philadelphia: Saunders, W.B.; 1999:457–470.
-
- Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. - PubMed
-
- Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. - PubMed
-
- Haggstrom J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

