Tumor proliferation and diffusion on percolation clusters
- PMID: 27678112
- PMCID: PMC5059599
- DOI: 10.1007/s10867-016-9427-2
Tumor proliferation and diffusion on percolation clusters
Abstract
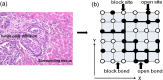
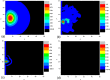
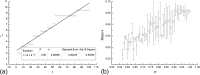
We study in silico the influence of host tissue inhomogeneity on tumor cell proliferation and diffusion by simulating the mobility of a tumor on percolation clusters with different homogeneities of surrounding tissues. The proliferation and diffusion of a tumor in an inhomogeneous tissue could be characterized in the framework of the percolation theory, which displays similar thresholds (0.54, 0.44, and 0.37, respectively) for tumor proliferation and diffusion in three kinds of lattices with 4, 6, and 8 connecting near neighbors. Our study reveals the existence of a critical transition concerning the survival and diffusion of tumor cells with leaping metastatic diffusion movement in the host tissues. Tumor cells usually flow in the direction of greater pressure variation during their diffusing and infiltrating to a further location in the host tissue. Some specific sites suitable for tumor invasion were observed on the percolation cluster and around these specific sites a tumor can develop into scattered tumors linked by some advantage tunnels that facilitate tumor invasion. We also investigate the manner that tissue inhomogeneity surrounding a tumor may influence the velocity of tumor diffusion and invasion. Our simulation suggested that invasion of a tumor is controlled by the homogeneity of the tumor microenvironment, which is basically consistent with the experimental report by Riching et al. as well as our clinical observation of medical imaging. Both simulation and clinical observation proved that tumor diffusion and invasion into the surrounding host tissue is positively correlated with the homogeneity of the tissue.
Keywords: Inhomogeneity; Invasive and metastatic diffusion; Percolation; Reaction–diffusion systems; Tumor.
Conflict of interest statement
Compliance with ethical standards Ethical approval This study did not involve any human experiments or treatment processes. The physiological and clinical images of tumors were acquired from ordinary medical examinations of patients at the Sun Yat-sen University Cancer Center. These examinations were carried out for therapy only and no additional drugs or measures were used. This study was approved by the ethics committee of the Sun Yat-sen University Cancer Center and every effort was made to maximize the protection of the patients’ privacy (e.g., anonymous analysis of data). The research materials and results were used for scientific purposes without any conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Figures








Similar articles
-
The anomalous diffusion of a tumor invading with different surrounding tissues.PLoS One. 2014 Oct 13;9(10):e109784. doi: 10.1371/journal.pone.0109784. eCollection 2014. PLoS One. 2014. PMID: 25310134 Free PMC article.
-
Threshold of hierarchical percolating systems.Phys Rev E Stat Nonlin Soft Matter Phys. 2012 Feb;85(2 Pt 1):021109. doi: 10.1103/PhysRevE.85.021109. Epub 2012 Feb 7. Phys Rev E Stat Nonlin Soft Matter Phys. 2012. PMID: 22463155
-
Lateral diffusion of molecules in two-component lipid bilayer: a Monte Carlo simulation study.J Phys Chem B. 2005 Apr 21;109(15):7373-86. doi: 10.1021/jp045669x. J Phys Chem B. 2005. PMID: 16851844
-
The tumor ecosystem regulates the roads for invasion and metastasis.Clin Res Hepatol Gastroenterol. 2011 Nov;35(11):714-9. doi: 10.1016/j.clinre.2011.05.003. Epub 2011 Jun 14. Clin Res Hepatol Gastroenterol. 2011. PMID: 21676670 Review.
-
Interaction of tumor cells with the extracellular matrix.Revis Biol Celular. 1987;12:1-113. Revis Biol Celular. 1987. PMID: 2447614 Review.
Cited by
-
In vivo mimicking model for solid tumor towards hydromechanics of tissue deformation and creation of necrosis.J Biol Phys. 2018 Sep;44(3):361-400. doi: 10.1007/s10867-018-9496-5. Epub 2018 May 28. J Biol Phys. 2018. PMID: 29808371 Free PMC article.
References
-
- Stewart BW, Wild CP, editors. World cancer report 2014. Lyon: IARC; 2014.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

