13C and 15N natural isotope abundance reflects breast cancer cell metabolism
- PMID: 27678172
- PMCID: PMC5039687
- DOI: 10.1038/srep34251
13C and 15N natural isotope abundance reflects breast cancer cell metabolism
Abstract
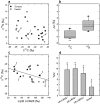
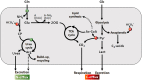
Breast cancer is the most common cancer in women worldwide. Despite the information provided by anatomopathological assessment and molecular markers (such as receptor expression ER, PR, HER2), breast cancer therapies and prognostics depend on the metabolic properties of tumor cells. However, metabolomics have not provided a robust and congruent biomarker yet, likely because individual metabolite contents are insufficient to encapsulate all of the alterations in metabolic fluxes. Here, we took advantage of natural 13C and 15N isotope abundance to show there are isotopic differences between healthy and cancer biopsy tissues or between healthy and malignant cultured cell lines. Isotope mass balance further suggests that these differences are mostly related to lipid metabolism, anaplerosis and urea cycle, three pathways known to be impacted in malignant cells. Our results demonstrate that the isotope signature is a good descriptor of metabolism since it integrates modifications in C partitioning and N excretion altogether. Our present study is thus a starting point to possible clinical applications such as patient screening and biopsy characterization in every cancer that is associated with metabolic changes.
Figures


References
-
- Graham D. Y. et al.. Campylobacter pylori detected noninvasively by the 13C-urea breath test. The Lancet 1, 1174–1177 (1987). - PubMed
-
- Schmidt H.-L., Robins R. J. & Werner R. A. Multi-factorial in vivo stable isotope fractionation: causes, correlations, consequences and applications. Isotopes Environ Health Stud 51, 155–199 (2015). - PubMed
-
- Bartstow T. J., Cooper D. M., Epstein S. & Wasserman K. Changes in 13CO2/12CO2 consequent to exercise and hypoxia. J Appl Physiol 66, 936–942 (1989). - PubMed
-
- Perkins S. E. & Speakman J. R. Measuring natural abundance of 13C in respired CO2: variability and implications for non-invasive dietary analysis. Funct Ecol 15, 791–797 (2001).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

