Time-resolved dual RNA-seq reveals extensive rewiring of lung epithelial and pneumococcal transcriptomes during early infection
- PMID: 27678244
- PMCID: PMC5039909
- DOI: 10.1186/s13059-016-1054-5
Time-resolved dual RNA-seq reveals extensive rewiring of lung epithelial and pneumococcal transcriptomes during early infection
Abstract
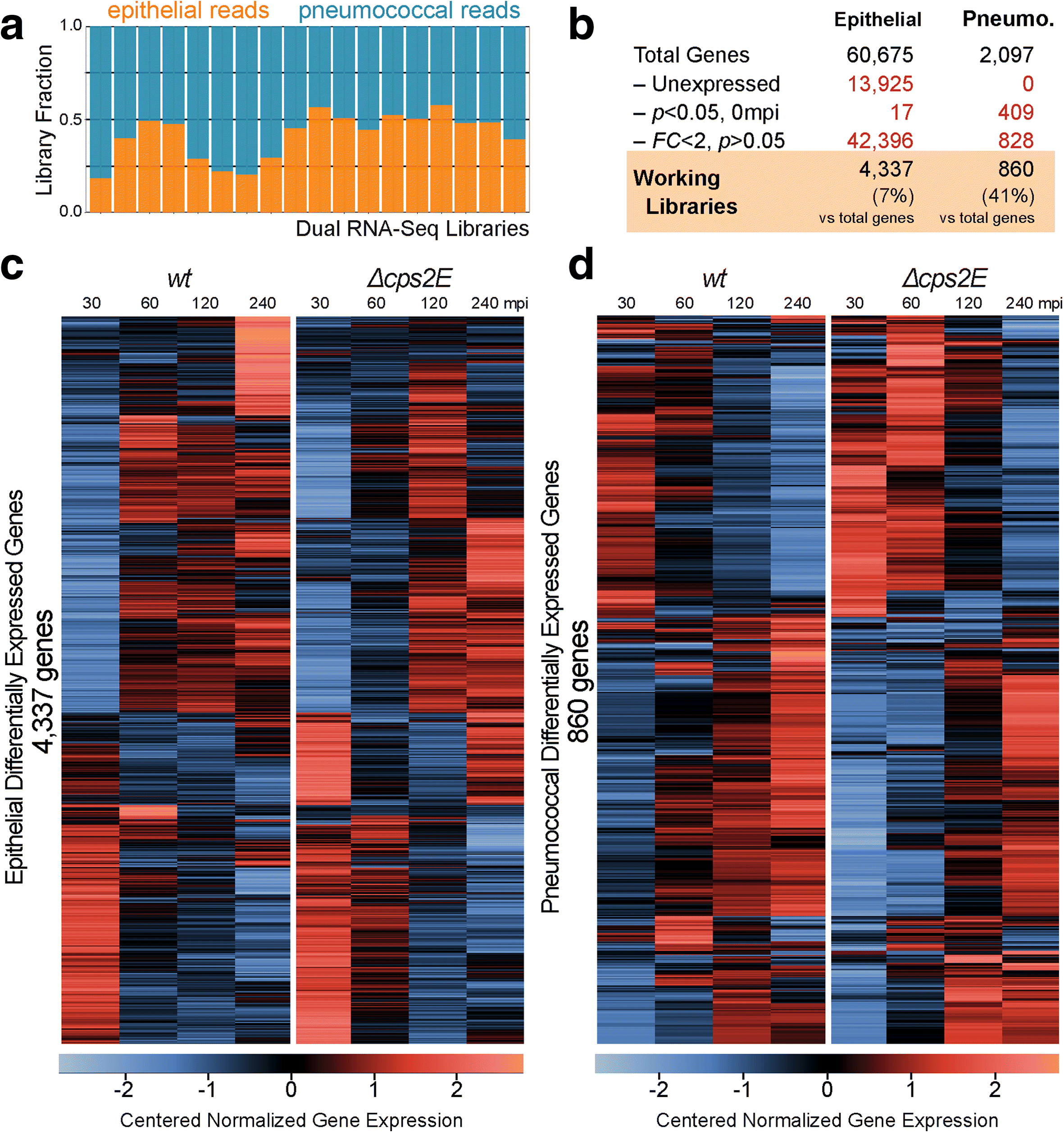
Background: Streptococcus pneumoniae, the pneumococcus, is the main etiological agent of pneumonia. Pneumococcal infection is initiated by bacterial adherence to lung epithelial cells. The exact transcriptional changes occurring in both host and microbe during infection are unknown. Here, we developed a time-resolved infection model of human lung alveolar epithelial cells by S. pneumoniae and assess the resulting transcriptome changes in both organisms simultaneously by using dual RNA-seq.
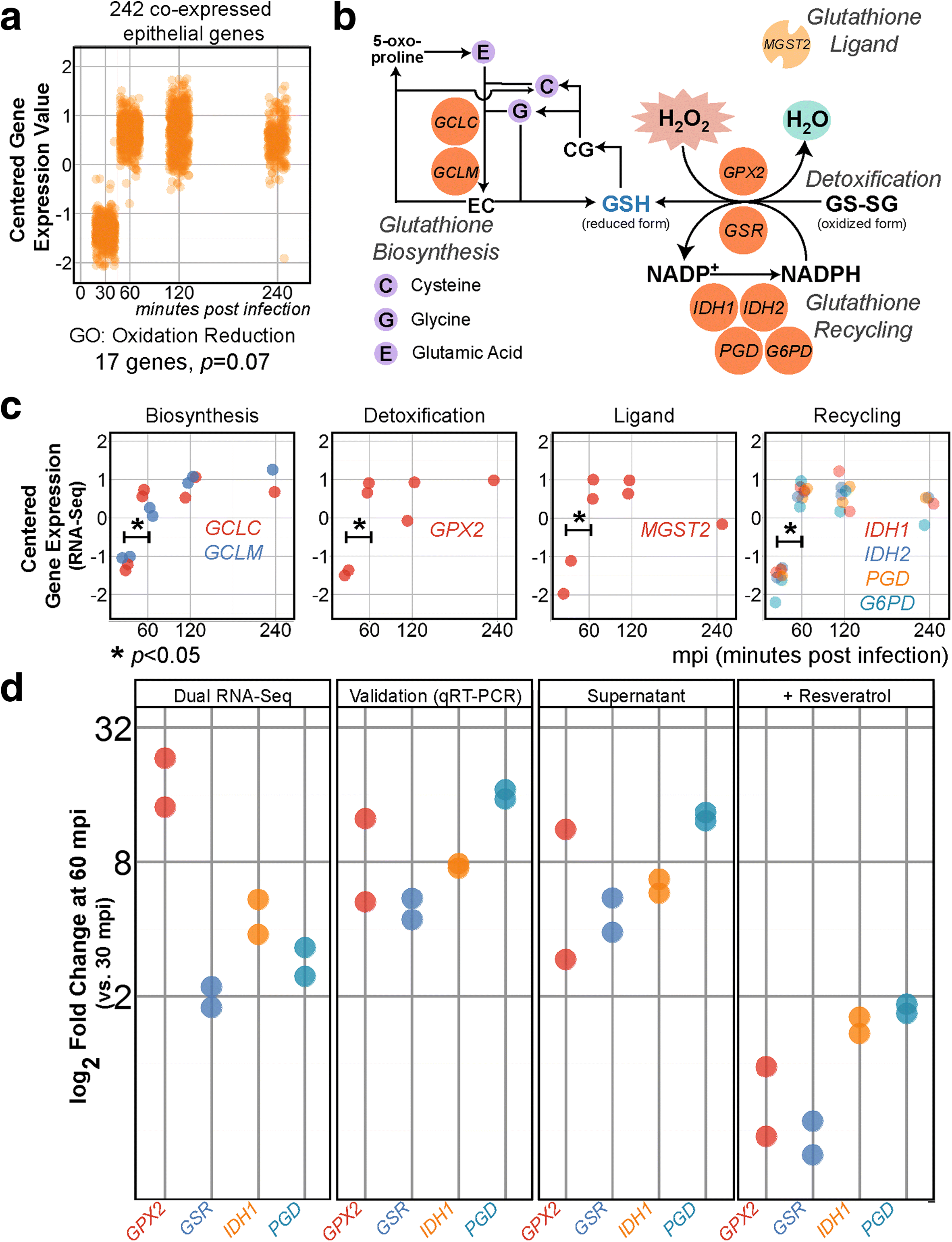
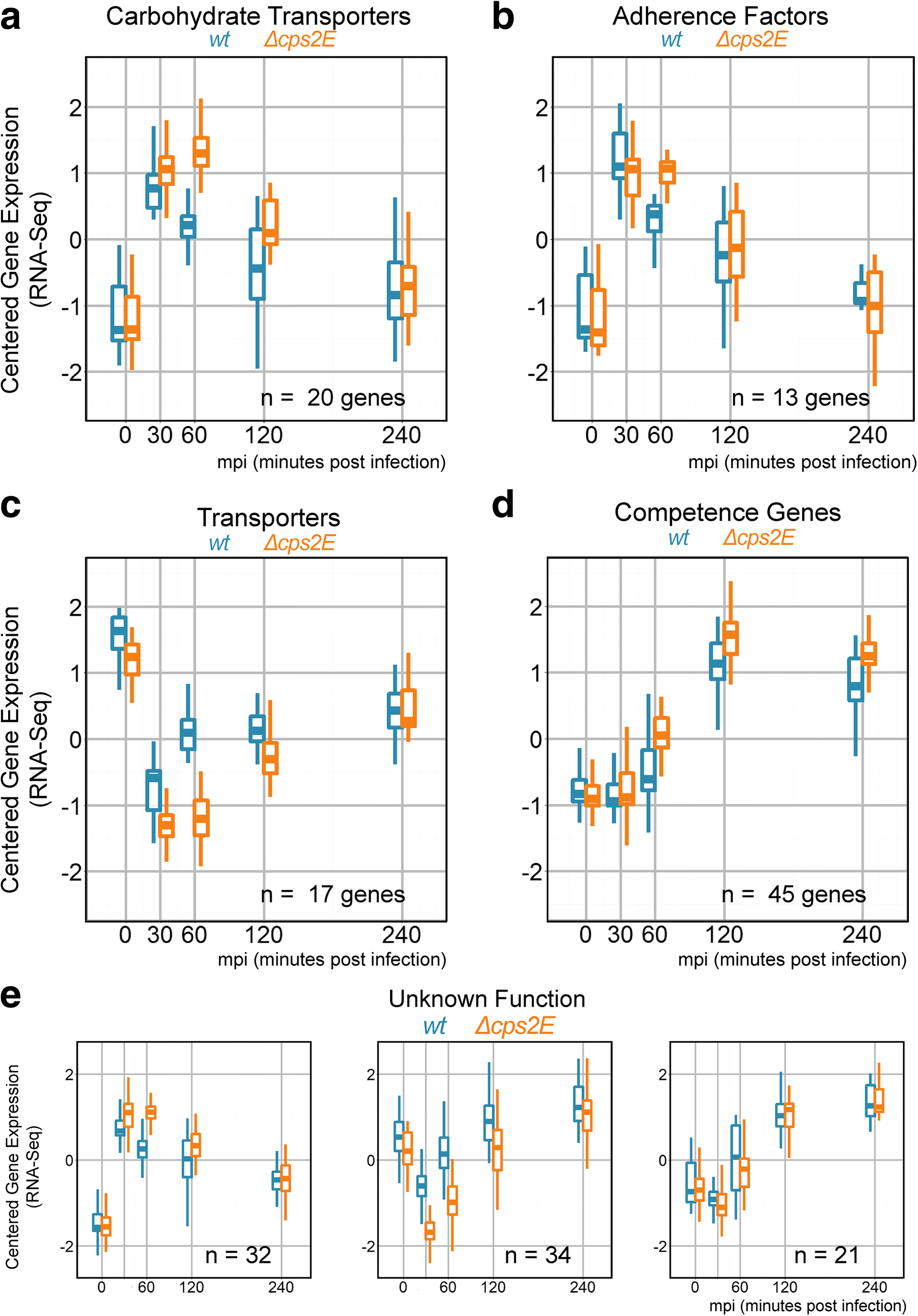
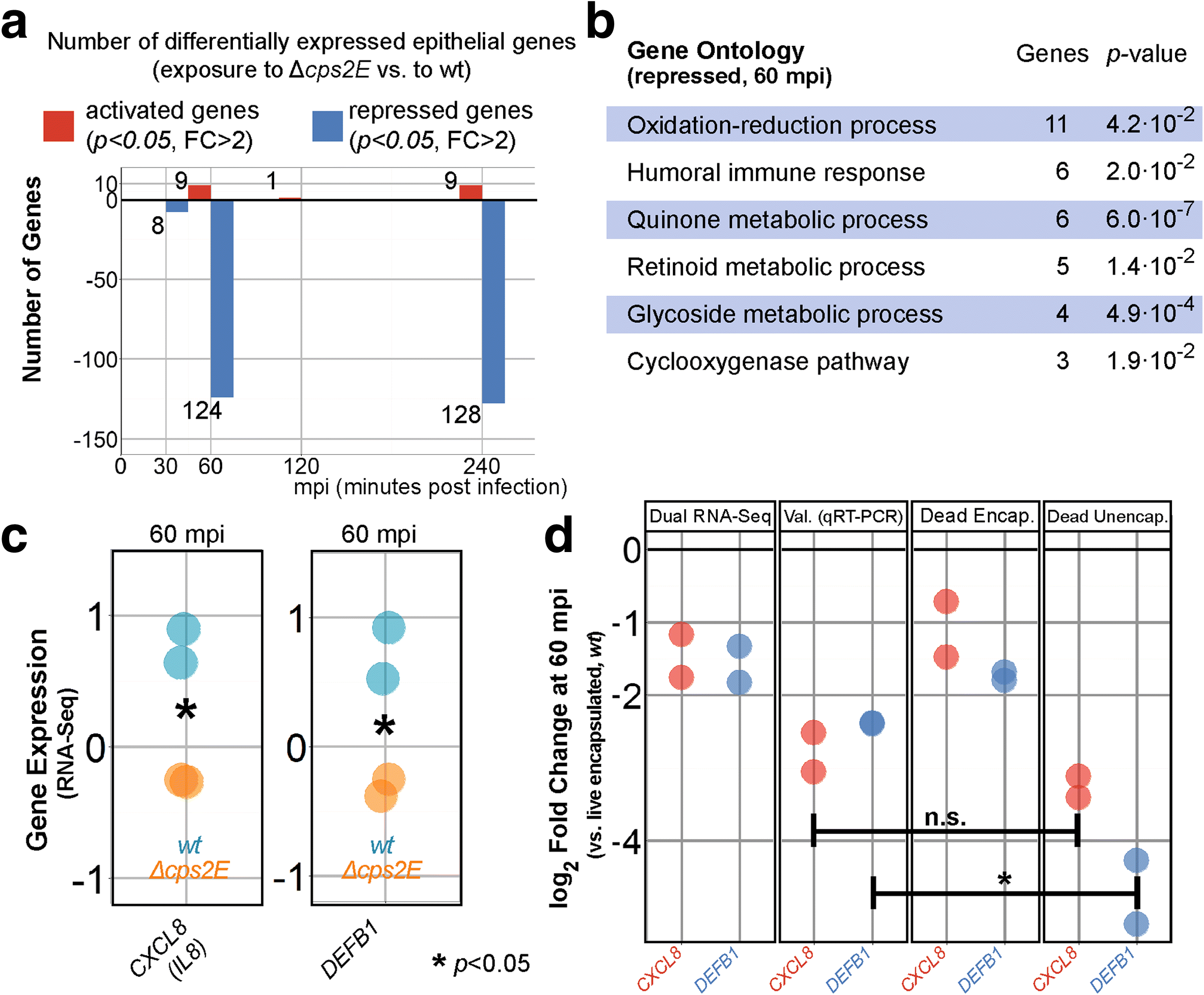
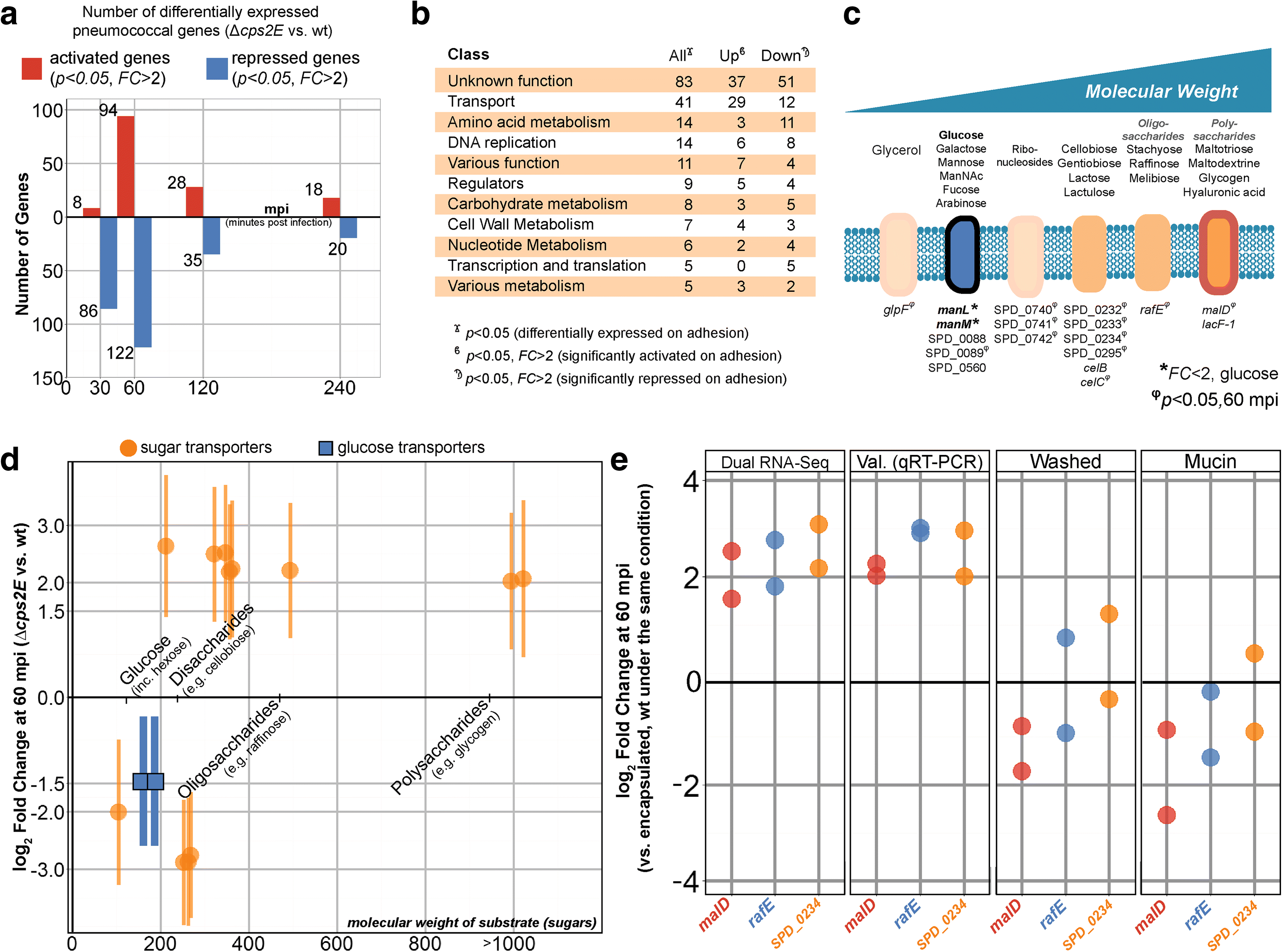
Results: Functional analysis of the time-resolved dual RNA-seq data identifies several features of pneumococcal infection. For instance, we show that the glutathione-dependent reactive oxygen detoxification pathway in epithelial cells is activated by reactive oxygen species produced by S. pneumoniae. Addition of the antioxidant resveratrol during infection abates this response. At the same time, pneumococci activate the competence regulon during co-incubation with lung epithelial cells. By comparing transcriptional changes between wild-type encapsulated and mutant unencapsulated pneumococci, we demonstrate that adherent pneumococci, but not free-floating bacteria, repress innate immune responses in epithelial cells including expression of the chemokine IL-8 and the production of antimicrobial peptides. We also show that pneumococci activate several sugar transporters in response to adherence to epithelial cells and demonstrate that this activation depends on host-derived mucins.
Conclusions: We provide a dual-transcriptomics overview of early pneumococcal infection in a time-resolved manner, providing new insights into host-microbe interactions. To allow easy access to the data by the community, a web-based platform was developed ( http://dualrnaseq.molgenrug.nl ). Further database exploration may expand our understanding of epithelial-pneumococcal interaction, leading to novel antimicrobial strategies.
Keywords: Adherence; Competence; Dual RNA-seq; Host-microbe interaction; Lung epithelial cells; Streptococcus pneumoniae; Systems biology; Transcriptomics.
Figures


References
-
- Hammerschmidt S, Bergmann S, Paterson GK, Mitchell TJ. Community-acquired pneumonia, Birkhäuser advances in infectious diseases. Basel: Birkhäuser; 2007. Pathogenesis of Streptococcus pneumoniae infections: adaptive immunity, innate immunity, cell biology, virulence factors; pp. 139–81.
-
- Bootsma HJ, Egmont-Petersen M, Hermans PWM. Analysis of the in vitro transcriptional response of human pharyngeal epithelial cells to adherent Streptococcus pneumoniae: evidence for a distinct response to encapsulated strains. Infect Immun. 2007;75(11):5489–99. doi: 10.1128/IAI.01823-06. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

