Genetic and Pharmacologic Inhibition of the Chemokine Receptor CXCR2 Prevents Experimental Hypertension and Vascular Dysfunction
- PMID: 27678262
- PMCID: PMC5084654
- DOI: 10.1161/CIRCULATIONAHA.115.020754
Genetic and Pharmacologic Inhibition of the Chemokine Receptor CXCR2 Prevents Experimental Hypertension and Vascular Dysfunction
Abstract
Background: The recruitment of leukocytes to the vascular wall is a key step in hypertension development. Chemokine receptor CXCR2 mediates inflammatory cell chemotaxis in several diseases. However, the role of CXCR2 in hypertension development and the underlying mechanisms remain unknown.
Methods: Angiotensin II (490 ng·kg-1·min-1) or deoxycorticosterone acetate (DOCA) salt-induced mouse hypertensive models in genetic ablation, pharmacologic inhibition of CXCR2, and adoptive bone marrow transfer mice were used to determine the role of CXCR2 in hypertension (measured by radiotelemetry and tail-cuff system), inflammation (verified by flow cytometry and quantitative real-time polymerase chain reaction [PCR] analysis), vascular remodeling (studied by haematoxylin and eosin and Masson's trichrome staining), vascular dysfunction (assessed by aortic ring), and oxidative stress (indicated by nicotinamide adenine dinucleotide phosphate [NADPH] oxidase activity, dihydroethidium staining, and quantitative real-time PCR analysis). Moreover, the blood CXCR2+ cells in normotensive controls and hypertension patients were analyzed by flow cytometry.
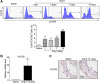
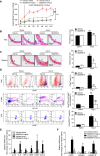
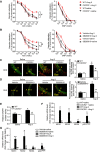
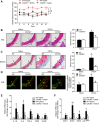
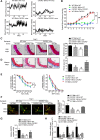
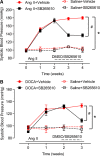
Results: Angiotensin II significantly upregulated the expression of CXCR2 mRNA and protein and increased the number of CD45+ CXCR2+ cells in mouse aorta (n=8 per group). Selective CXCR2 knockout (CXCR2-/-) or pharmacological inhibition of CXCR2 markedly reduced angiotensin II- or DOCA-salt-induced blood pressure elevation, aortic thickness and collagen deposition, accumulation of proinflammatory cells into the vascular wall, and expression of cytokines (n=8 per group). CXCR2 inhibition also ameliorated angiotensin II-induced vascular dysfunction and reduced vascular superoxide formation, NADPH activity, and expression of NADPH oxidase subunits (n=6 per group). Bone marrow reconstitution of wild-type mice with CXCR2-/- bone marrow cells also significantly abolished angiotensin II-induced responses (n=6 per group). It is important to note that CXCR2 blockade reversed established hypertension induced by angiotensin II or DOCA-salt challenge (n=10 per group). Furthermore, we demonstrated that CXCR2+ proinflammatory cells were higher in hypertensive patients (n=30) compared with normotensive individuals (n=20).
Conclusions: Infiltration of CXCR2+ cells plays a pathogenic role in arterial hypertension and vascular dysfunction. Inhibition of CXCR2 pathway may represent a novel therapeutic approach to treat hypertension.
Keywords: CXCR2; chemoreceptor; hypertension; inflammation; oxidant stress.
© 2016 The Authors.
Figures


Comment in
-
Is Hypertension a Bone Marrow Disease?Circulation. 2016 Nov 1;134(18):1369-1372. doi: 10.1161/CIRCULATIONAHA.116.024520. Epub 2016 Sep 27. Circulation. 2016. PMID: 27678263 Free PMC article. No abstract available.
References
-
- Rodríguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA, Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol. 2014;10:56–62. doi: 10.1038/nrneph.2013.248. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

