A Novel Compound ARN-3236 Inhibits Salt-Inducible Kinase 2 and Sensitizes Ovarian Cancer Cell Lines and Xenografts to Paclitaxel
- PMID: 27678456
- PMCID: PMC5436602
- DOI: 10.1158/1078-0432.CCR-16-1562
A Novel Compound ARN-3236 Inhibits Salt-Inducible Kinase 2 and Sensitizes Ovarian Cancer Cell Lines and Xenografts to Paclitaxel
Abstract
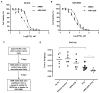
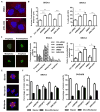
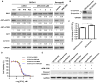
Purpose: Salt-inducible kinase 2 (SIK2) is a centrosome kinase required for mitotic spindle formation and a potential target for ovarian cancer therapy. Here, we examine the effects of a novel small-molecule SIK2 inhibitor, ARN-3236, on sensitivity to paclitaxel in ovarian cancer.Experimental Design: SIK2 expression was determined in ovarian cancer tissue samples and cell lines. ARN-3236 was tested for its efficiency to inhibit growth and enhance paclitaxel sensitivity in cultures and xenografts of ovarian cancer cell lines. SIK2 siRNA and ARN-3236 were compared for their ability to produce nuclear-centrosome dissociation, inhibit centrosome splitting, block mitotic progression, induce tetraploidy, trigger apoptotic cell death, and reduce AKT/survivin signaling.Results: SIK2 is overexpressed in approximately 30% of high-grade serous ovarian cancers. ARN-3236 inhibited the growth of 10 ovarian cancer cell lines at an IC50 of 0.8 to 2.6 μmol/L, where the IC50 of ARN-3236 was inversely correlated with endogenous SIK2 expression (Pearson r = -0.642, P = 0.03). ARN-3236 enhanced sensitivity to paclitaxel in 8 of 10 cell lines, as well as in SKOv3ip (P = 0.028) and OVCAR8 xenografts. In at least three cell lines, a synergistic interaction was observed. ARN-3236 uncoupled the centrosome from the nucleus in interphase, blocked centrosome separation in mitosis, caused prometaphase arrest, and induced apoptotic cell death and tetraploidy. ARN-3236 also inhibited AKT phosphorylation and attenuated survivin expression.Conclusions: ARN-3236 is the first orally available inhibitor of SIK2 to be evaluated against ovarian cancer in preclinical models and shows promise in inhibiting ovarian cancer growth and enhancing paclitaxel chemosensitivity. Clin Cancer Res; 23(8); 1945-54. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



References
-
- Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18:106–15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

