Development of a Smart Pump for Monitoring and Controlling Intraocular Pressure
- PMID: 27679446
- PMCID: PMC5364042
- DOI: 10.1007/s10439-016-1735-y
Development of a Smart Pump for Monitoring and Controlling Intraocular Pressure
Abstract
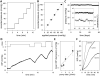
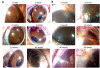
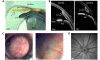
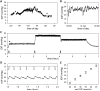
Animal models of ocular hypertension are important for glaucoma research but come with experimental costs. Available methods of intraocular pressure (IOP) elevation are not always successful, the amplitude and time course of IOP changes are unpredictable and irreversible, and IOP measurement by tonometry is laborious. Here we present a novel system for monitoring and controlling IOP without these limitations. It consists of a cannula implanted in the anterior chamber of the eye, a pressure sensor that continually measures IOP, and a bidirectional pump driven by control circuitry that can infuse or withdraw fluid to hold IOP at user-desired levels. A portable version was developed for tethered use on rats. We show that rat eyes can be cannulated for months without causing significant anatomical or physiological damage although the animal and its eyes freely move. We show that the system measures IOP with <0.7 mmHg resolution and <0.3 mmHg/month drift and can maintain IOP within a user-specified window of desired levels for any duration necessary. We conclude that the system is ready for cage- or bench-side applications. The results lay the foundation for an implantable version that would give glaucoma researchers unprecedented knowledge and control of IOP in rats and potentially larger animals.
Keywords: Closed loop control; Eye; Glaucoma; Implant; Rat; Telemetry.
Figures




References
-
- Abrams LS, Vitale S, Jampel HD. Comparison of three tonometers for measuring intraocular pressure in rabbits. Invest. Ophthalmol. Vis. Sci. 1996;37:940–944. - PubMed
-
- Akaishi T, Ishida N, Shimazaki A, Hara H, Kuwayama Y. Continuous monitoring of circadian variations in intraocular pressure by telemetry system throughout a 12-week treatment with timolol maleate in rabbits. J. Ocul. Pharmacol. Therap. 2005;21:436–444. - PubMed
-
- Akula JD, Favazza TL, Mocko JA, Benador IY, Asturias AL, Kleinman MS, Hansen RM, Fulton AB. The anatomy of the rat eye with oxygen-induced retinopathy. Doc. Ophthalmol. 2010;120:41–50. - PubMed
-
- Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J. Glaucoma. 2000;9:134–142. - PubMed
-
- Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmol. 2007;114:205–209. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

