Regional and Gender Study of Neuronal Density in Brain during Aging and in Alzheimer's Disease
- PMID: 27679571
- PMCID: PMC5020132
- DOI: 10.3389/fnagi.2016.00213
Regional and Gender Study of Neuronal Density in Brain during Aging and in Alzheimer's Disease
Abstract
Background: Learning processes or language development are only some of the cognitive functions that differ qualitatively between men and women. Gender differences in the brain structure seem to be behind these variations. Indeed, this sexual dimorphism at neuroanatomical level is accompanied unequivocally by differences in the way that aging and neurodegenerative diseases affect men and women brains.
Objective: The aim of this study is the analysis of neuronal density in four areas of the hippocampus, and entorhinal and frontal cortices to analyze the possible gender influence during normal aging and in Alzheimer's disease (AD).
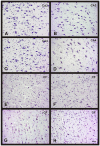
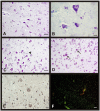
Methods: Human brain tissues of different age and from both sexes, without neurological pathology and with different Braak's stages of AD, were studied. Neuronal density was quantified using the optical dissector.
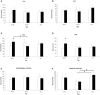
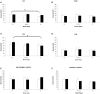
Results: Our results showed the absence of a significant neuronal loss during aging in non-pathological brains in both sexes. However, we have demonstrated specific punctual significant variations in neuronal density related with the age and gender in some regions of these brains. In fact, we observed a higher neuronal density in CA3 and CA4 hippocampal areas of non-pathological brains of young men compared to women. During AD, we observed a negative correlation between Braak's stages and neuronal density in hippocampus, specifically in CA1 for women and CA3 for men, and in frontal cortex for both, men and women.
Conclusion: Our data demonstrated a sexual dimorphism in the neuronal vulnerability to degeneration suggesting the need to consider the gender of the individuals in future studies, regarding neuronal loss in aging and AD, in order to avoid problems in interpreting data.
Keywords: Alzheimer's disease; age; entorhinal cortex; frontal cortex; hippocampus; human; sexual dimorphism.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

