Isolation and Functional Characterization of a Lycopene β-cyclase Gene Promoter from Citrus
- PMID: 27679644
- PMCID: PMC5020073
- DOI: 10.3389/fpls.2016.01367
Isolation and Functional Characterization of a Lycopene β-cyclase Gene Promoter from Citrus
Abstract
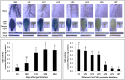
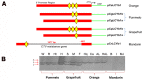
Lycopene β-cyclases are key enzymes located at the branch point of the carotenoid biosynthesis pathway. However, the transcriptional regulatory mechanisms of LCYb1 in citrus with abundant carotenoid accumulation are still unclear. To understand the molecular basis of CsLCYb1 expression, we isolated and functionally characterized the 5' upstream sequences of CsLCYb1 from citrus. The full-length CsLCYb1 promoter and a series of its 5' deletions were fused to the β-glucuronidase (GUS) reporter gene and transferred into different plants (tomato, Arabidopsis and citrus callus) to test the promoter activities. The results of all transgenic species showed that the 1584 bp upstream region from the translational start site displayed maximal promoter activity, and the minimal promoter containing 746 bp upstream sequences was sufficient for strong basal promoter activity. Furthermore, the CsLCYb1 promoter activity was developmentally and tissue-specially regulated in transgenic Arabidopsis, and it was affected by multiple hormones and environmental cues in transgenic citrus callus under various treatments. Finer deletion analysis identified an enhancer element existing as a tandem repeat in the promoter region between -574 to -513 bp and conferring strong promoter activity. The copy numbers of the enhancer element differed among various citrus species, leading to the development of a derived simple sequence repeat marker to distinguish different species. In conclusion, this study elucidates the expression characteristics of the LCYb1 promoter from citrus and further identifies a novel enhancer element required for the promoter activity. The characterized promoter fragment would be an ideal candidate for genetic engineering and seeking of upstream trans-acting elements.
Keywords: carotenoid; cis-element; citrus; enhancer; lycopene β-cyclase; promoter.
Figures





References
-
- Alquezar B., Rodrigo M. J., Lado J., Zacarías L. (2013). A comparative physiological and transcriptional study of carotenoid biosynthesis in white and red grapefruit (Citrus paradisi Macf.). Tree Genet. Genomes 9 1257–1269. 10.1007/s11295-013-0635-7 - DOI
-
- Banerjee J., Sahoo D. K., Raha S., Sarkar S., Dey N., Maiti I. B. (2015). A region Containing an as-1 element of Dahlia Mosaic Virus (DaMV) subgenomic transcript promoter plays a key role in green tissue-and root-specific expression in plants. Plant Mol. Biol. Rep. 33 532–556. 10.1007/s11105-014-0766-5 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

