A π-gel scaffold for assembling fullerene to photoconducting supramolecular rods
- PMID: 27679815
- PMCID: PMC5035126
- DOI: 10.1126/sciadv.1600142
A π-gel scaffold for assembling fullerene to photoconducting supramolecular rods
Abstract
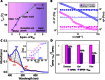
Nonequilibrium self-assembly of molecules holds a huge prospect as a tool for obtaining new-generation materials for future applications. Crystallization of neutral molecules within a supramolecular gel matrix is one example in which two nonequilibrium processes occur orthogonal to each other. On the other hand, electronically interacting donor-acceptor two-component systems are expected to form phase-miscible hybrid systems. Contrary to the expectation, we report the behavior of a π-gel, derived from oligo(p-phenylenevinylene), OPVA, as a scaffold for the phase separation and crystallization of fullerene (C60) to supramolecular rods with increased transient photoconductivity (φƩμmax = 2.4 × 10-4 cm2 V-1 s-1). The C60 supramolecular rods in the π-gel medium exhibited high photocurrent in comparison to C60 loaded in a non-π-gel medium. This finding provides an opportunity for large-scale preparation of micrometer-sized photoconducting rods of fullerenes for device application.
Keywords: Self-assembly; crystallization; fullerene; gels; p-n heterojunctions; photoconductivity; self-sorting.
Figures





References
-
- Kumar D. K., Steed J. W., Supramolecular gel phase crystallization: Orthogonal self-assembly under non-equilibrium conditions. Chem. Soc. Rev. 43, 2080–2088 (2014). - PubMed
-
- Mattia E., Otto S., Supramolecular systems chemistry. Nat. Nanotechnol. 10, 111–119 (2015). - PubMed
-
- Korevaar P. A., George S. J., Markvoort A. J., Smulders M. M. J., Hilbers P. A. J., Schenning A. P. H. J., De Greef T. F. A., Meijer E. W., Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012). - PubMed
-
- Li J.-L., Liu X.-Y., Architecture of supramolecular soft functional materials: From understanding to micro-/nanoscale engineering. Adv. Funct. Mater. 20, 3196–3216 (2010).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

