Structural and quantum chemical analysis of exciton coupling in homo- and heteroaggregate stacks of merocyanines
- PMID: 27680284
- PMCID: PMC5056423
- DOI: 10.1038/ncomms12949
Structural and quantum chemical analysis of exciton coupling in homo- and heteroaggregate stacks of merocyanines
Abstract
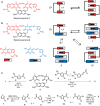
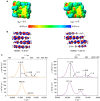
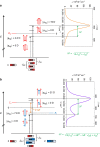
Exciton coupling is of fundamental importance and determines functional properties of organic dyes in (opto-)electronic and photovoltaic devices. Here we show that strong exciton coupling is not limited to the situation of equal chromophores as often assumed. Quadruple dye stacks were obtained from two bis(merocyanine) dyes with same or different chromophores, respectively, which dimerize in less-polar solvents resulting in the respective homo- and heteroaggregates. The structures of the quadruple dye stacks were assigned by NMR techniques and unambiguously confirmed by single-crystal X-ray analysis. The heteroaggregate stack formed from the bis(merocyanine) bearing two different chromophores exhibits remarkably different ultraviolet/vis absorption bands compared with those of the homoaggregate of the bis(merocyanine) comprising two identical chromophores. Quantum chemical analysis based on an extension of Kasha's exciton theory appropriately describes the absorption properties of both types of stacks revealing strong exciton coupling also between different chromophores within the heteroaggregate.
Figures



References
-
- Kasha M., Rawls H. R. & El-Bayoumi M. A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 11, 371–392 (1965).
-
- Davydov A. S. The theory of molecular excitons. Sov. Phys. Usp. 7, 145–178 (1964).
-
- Bhosale R. et al.. Zipper assembly of SHJ photosystems: focus on red naphthalenediimides, optoelectronic finetuning and topological matching. Chem. Sci. 1, 357–368 (2010).
-
- Wasielewski M. R. Self-assembly strategies for integrating light harvesting and charge separation in artificial photosynthetic systems. Acc. Chem. Res. 42, 1910–1921 (2009). - PubMed
-
- Praveen V. K., Ranjith C., Bandini E., Ajayaghosh A. & Armaroli N. Oligo(phenylenevinylene) hybrids and self-assemblies: versatile materials for excitation energy transfer. Chem. Soc. Rev. 43, 4222–4242 (2014). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

