Aromatic thiol-mediated cleavage of N-O bonds enables chemical ubiquitylation of folded proteins
- PMID: 27680493
- PMCID: PMC5056422
- DOI: 10.1038/ncomms12979
Aromatic thiol-mediated cleavage of N-O bonds enables chemical ubiquitylation of folded proteins
Abstract
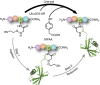
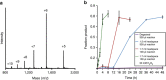
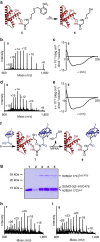
Access to protein substrates homogenously modified by ubiquitin (Ub) is critical for biophysical and biochemical investigations aimed at deconvoluting the myriad biological roles for Ub. Current chemical strategies for protein ubiquitylation, however, employ temporary ligation auxiliaries that are removed under harsh denaturing conditions and have limited applicability. We report an unprecedented aromatic thiol-mediated N-O bond cleavage and its application towards native chemical ubiquitylation with the ligation auxiliary 2-aminooxyethanethiol. Our interrogation of the reaction mechanism suggests a disulfide radical anion as the active species capable of cleaving the N-O bond. The successful semisynthesis of full-length histone H2B modified by the small ubiquitin-like modifier-3 (SUMO-3) protein further demonstrates the generalizability and compatibility of our strategy with folded proteins.
Figures



References
-
- Sharp P. M. & Li W. H. Molecular evolution of ubiquitin genes. Trends Ecol. Evol. 2, 328–332 (1987). - PubMed
-
- Van der Veen A. G. & Ploegh H. L. Ubiquitin-like proteins. Annu. Rev. Biochem. 81, 323–357 (2012). - PubMed
-
- Komander D. & Rape M. The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

