Novel biomarkers that assist in accurate discrimination of squamous cell carcinoma from adenocarcinoma of the lung
- PMID: 27681076
- PMCID: PMC5041559
- DOI: 10.1186/s12885-016-2792-1
Novel biomarkers that assist in accurate discrimination of squamous cell carcinoma from adenocarcinoma of the lung
Abstract
Background: Targeted therapies based on the molecular and histological features of cancer types are becoming standard practice. The most effective regimen in lung cancers is different between squamous cell carcinoma (SCC) and adenocarcinoma (AD). Therefore a precise diagnosis is crucial, but this has been difficult, particularly for poorly differentiated SCC (PDSCC) and AD without a lepidic growth component (non-lepidic AD). Biomarkers enabling a precise diagnosis are therefore urgently needed.
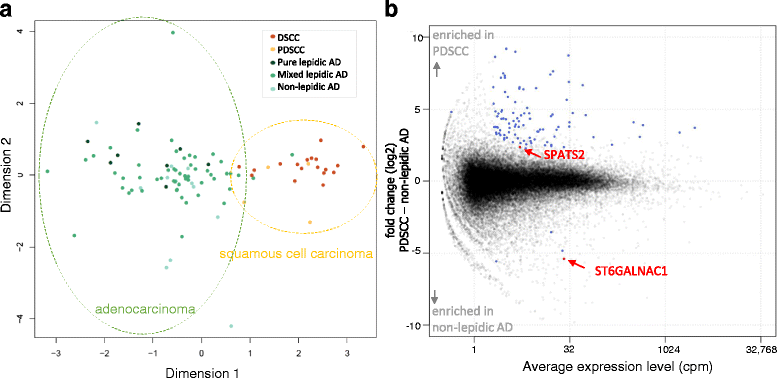
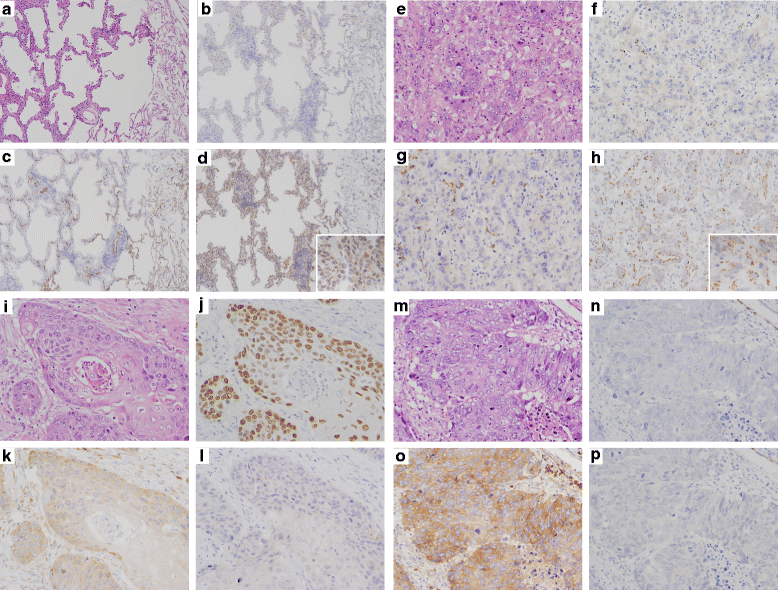
Methods: Cap Analysis of Gene Expression (CAGE) is a method used to quantify promoter activities across the whole genome by determining the 5' ends of capped RNA molecules with next-generation sequencing. We performed CAGE on 97 frozen tissues from surgically resected lung cancers (22 SCC and 75 AD), and confirmed the findings by immunohistochemical analysis (IHC) in an independent group (29 SCC and 45 AD).
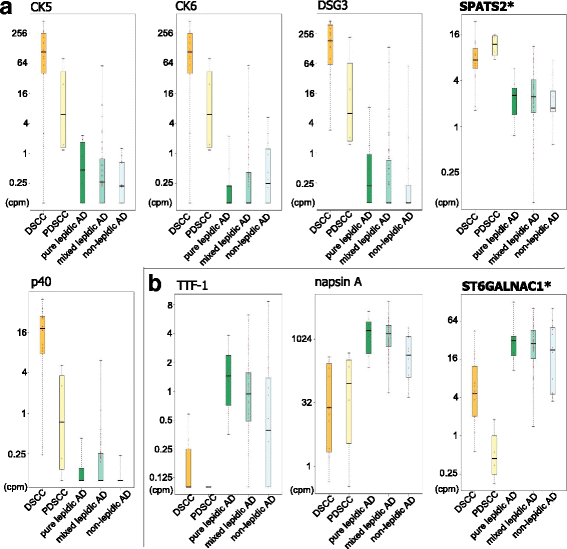
Results: Using the genome-wide promoter activity profiles, we confirmed that the expression of known molecular markers used in IHC for SCC (CK5, CK6, p40 and desmoglein-3) and AD (TTF-1 and napsin A) were different between SCC and AD. We identified two novel marker candidates, SPATS2 for SCC and ST6GALNAC1 for AD, as showing comparable performance and complementary utility to the known markers in discriminating PDSCC and non-lepidic AD. We subsequently confirmed their utility at the protein level by IHC in an independent group.
Conclusions: We identified two genes, SPATS2 and ST6GALNAC1, as novel complemental biomarkers discriminating SCC and AD. These findings will contribute to a more accurate diagnosis of NSCLC, which is crucial for precision medicine for lung cancer.
Figures

References
-
- Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

