The Splicing History of an mRNA Affects Its Level of Translation and Sensitivity to Cleavage by the Virion Host Shutoff Endonuclease during Herpes Simplex Virus Infections
- PMID: 27681125
- PMCID: PMC5110170
- DOI: 10.1128/JVI.01302-16
The Splicing History of an mRNA Affects Its Level of Translation and Sensitivity to Cleavage by the Virion Host Shutoff Endonuclease during Herpes Simplex Virus Infections
Abstract
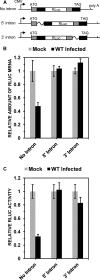
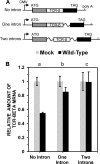
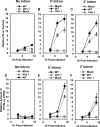
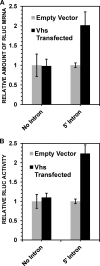
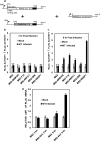
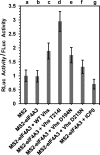
During lytic herpes simplex virus (HSV) infections, the virion host shutoff (Vhs) (UL41) endoribonuclease degrades many cellular and viral mRNAs. In uninfected cells, spliced mRNAs emerge into the cytoplasm bound by exon junction complexes (EJCs) and are translated several times more efficiently than unspliced mRNAs that have the same sequence but lack EJCs. Notably, most cellular mRNAs are spliced, whereas most HSV mRNAs are not. To examine the effect of splicing on gene expression during HSV infection, cells were transfected with plasmids harboring an unspliced renilla luciferase (RLuc) reporter mRNA or RLuc constructs with introns near the 5' or 3' end of the gene. After splicing of intron-containing transcripts, all three RLuc mRNAs had the same primary sequence. Upon infection in the presence of actinomycin D, spliced mRNAs were much less sensitive to degradation by copies of Vhs from infecting virions than were unspliced mRNAs. During productive infections (in the absence of drugs), RLuc was expressed at substantially higher levels from spliced than from unspliced mRNAs. Interestingly, the stimulatory effect of splicing on RLuc expression was significantly greater in infected than in uninfected cells. The translational stimulatory effect of an intron during HSV-1 infections could be replicated by artificially tethering various EJC components to an unspliced RLuc transcript. Thus, the splicing history of an mRNA, and the consequent presence or absence of EJCs, affects its level of translation and sensitivity to Vhs cleavage during lytic HSV infections.
Importance: Most mammalian mRNAs are spliced. In contrast, of the more than 80 mRNAs harbored by herpes simplex virus 1 (HSV-1), only 5 are spliced. In addition, synthesis of the immediate early protein ICP27 causes partial inhibition of pre-mRNA splicing, with the resultant accumulation of both spliced and unspliced versions of some mRNAs in the cytoplasm. A common perception is that HSV-1 infection necessarily inhibits the expression of spliced mRNAs. In contrast, this study demonstrates two instances in which pre-mRNA splicing actually enhances the synthesis of proteins from mRNAs during HSV-1 infections. Specifically, splicing stabilized an mRNA against degradation by copies of the Vhs endoribonuclease from infecting virions and greatly enhanced the amount of protein synthesized from spliced mRNAs at late times after infection. The data suggest that splicing, and the resultant presence of exon junction complexes on an mRNA, may play an important role in gene expression during HSV-1 infections.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Roizman B, Knipe DM, Whitley RJ. 2013. Herpes simplex viruses, p 1823–1897. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

