Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Randomized, Placebo-Controlled Study
- PMID: 27681442
- PMCID: PMC5312066
- DOI: 10.1038/npp.2016.224
Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Randomized, Placebo-Controlled Study
Abstract
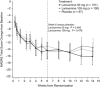
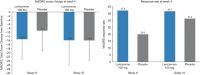
The objective of this study was to investigate the efficacy and safety of adjunctive lanicemine (NMDA channel blocker) in the treatment of major depressive disorder (MDD) over 12 weeks. This phase IIb, randomized, parallel-arm, double-blind, placebo-controlled study was conducted at 49 centers in four countries between December 2011 and August 2013 in 302 patients aged 18-70 years, meeting criteria for single episode or recurrent MDD and with a history of inadequate treatment response. Patients were required to be taking an allowed antidepressant for at least four weeks prior to screening. Patients were randomized equally to receive 15 double-blind intravenous infusions of adjunctive lanicemine 50 mg, lanicemine 100 mg, or saline over a 12-week course, in addition to ongoing antidepressant. The primary efficacy end point was change in Montgomery-Åsberg Depression Rating Scale (MADRS) total score from baseline to week 6. Secondary efficacy outcome variables included change in MADRS score from baseline to week 12, response and remission rates, and changes in Clinical Global Impression scale, Quick Inventory of Depressive Symptomology Self-Report score, and Sheehan Disability Scale score. Of 302 randomized patients, 240 (79.5%) completed treatment. Although lanicemine was generally well tolerated, neither dose was superior to placebo in reducing depressive symptoms on the primary end point or any secondary measures. There was no significant difference between lanicemine and placebo treatment on any outcome measures related to MDD. Post hoc analyses were performed to explore the possible effects of trial design and patient characteristics in accounting for the contrasting results with a previously reported trial.
Figures

References
-
- Berman RM, Fava M, Thase ME, Trivedi MH, Swanink R, McQuade RD et al (2009). Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr 14: 197–206. - PubMed
-
- Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK et al (2007). The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68: 843–853. - PubMed
-
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS et al (1998). Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11: 125–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

