Anti-Cancer Effect of Quercetin in Xenograft Models with EBV-Associated Human Gastric Carcinoma
- PMID: 27681719
- PMCID: PMC6274130
- DOI: 10.3390/molecules21101286
Anti-Cancer Effect of Quercetin in Xenograft Models with EBV-Associated Human Gastric Carcinoma
Abstract
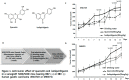
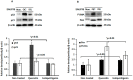
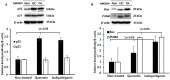
Licorice extracts have been widely used in herbal and folk medications. Glycyrrhiza contains diverse range of biological compounds including triterpenes (glycyrrhizin, glycyrrhizic acid) and flavonoids (quercetin, liquiritin, liquiritigenin, glabridin, licoricidin, isoliquiritigenin). The flavonoids in licorice are known to have strong anti-cancer activities. Quercetin, the most abundant flavonoid, has been shown to have anti-ulcer, anti-cancer, antioxidant, and anti-inflammatory properties. Latent Epstein-Barr virus (EBV) infection can lead to serious malignancies, such as, Burkitt's lymphoma, Hodgkin's disease and gastric carcinoma(GC), and (Epstein-Barr virus associated gastric carcinoma) EBVaGC is one of the most common EBV-associated cancers. In this study, the authors first examined the anti-cancer effects of quercetin and isoliquiritigenin in vivo xenograft animal models implanted with EBV(+) human gastric carcinoma (SNU719) or EBV(-) human gastric carcinoma (MKN74), and then explored the molecular mechanisms responsible for their anti-cancer activities. The results obtained showed that anti-cancer effect of quercetin was greater than isoliquiritigenin in mice injected with EBV(+) human gastric carcinoma (SNU719) cells. On the other hand, quercetin and isoliquiritigenin had similar anti-cancer effects in mice injected with EBV(-) human gastric carcinoma (MKN74) cells. Interestingly, quercetin inhibited EBV viral protein expressions, including EBNA-1 and LMP-2 proteins in tumor tissues from mice injected with EBV(+) human gastric carcinoma. Quercetin more effectively induced p53-dependent apoptosis than isoliquiritigenin in EBV(+) human gastric carcinoma, and this induction was correlated with increased expressions of the cleaved forms of caspase-3, -9, and Parp. In EBV(-)human gastric carcinoma (MKN74), both quercetin and isoliquiritigenin induced the expressions of p53, Bax, and Puma and the cleaved forms of caspase-3 and -9 and Parp at similar levels.
Keywords: EBV; SNU719; human gastric carcinoma; p53; quercetin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Quercetin-induced apoptosis prevents EBV infection.Oncotarget. 2015 May 20;6(14):12603-24. doi: 10.18632/oncotarget.3687. Oncotarget. 2015. PMID: 26059439 Free PMC article.
-
Quercetin Synergistically Inhibit EBV-Associated Gastric Carcinoma with Ganoderma lucidum Extracts.Molecules. 2019 Oct 24;24(21):3834. doi: 10.3390/molecules24213834. Molecules. 2019. PMID: 31653035 Free PMC article.
-
The interplay between Epstein-Bar virus (EBV) with the p53 and its homologs during EBV associated malignancies.Heliyon. 2019 Nov 14;5(11):e02624. doi: 10.1016/j.heliyon.2019.e02624. eCollection 2019 Nov. Heliyon. 2019. PMID: 31840114 Free PMC article. Review.
-
[Expression of Epstein-Barr virus genes in EBV-associated gastric carcinoma].Ai Zheng. 2004 Jul;23(7):782-7. Ai Zheng. 2004. PMID: 15248912 Chinese.
-
T cell recognition of Epstein-Barr virus associated lymphomas.Cancer Surv. 1992;13:53-80. Cancer Surv. 1992. PMID: 1330300 Review.
Cited by
-
Metabolomics Revealed the Tolerance and Growth Dynamics of Arbuscular Mycorrhizal Fungi (AMF) to Soil Salinity in Licorice.Plants (Basel). 2024 Sep 22;13(18):2652. doi: 10.3390/plants13182652. Plants (Basel). 2024. PMID: 39339628 Free PMC article.
-
Using glycyrrhizic acid to target sumoylation processes during Epstein-Barr virus latency.PLoS One. 2019 May 24;14(5):e0217578. doi: 10.1371/journal.pone.0217578. eCollection 2019. PLoS One. 2019. PMID: 31125383 Free PMC article.
-
Anti-Epstein-Barr Virus Activities of Flavones and Flavonols with Effects on Virus-Related Cancers.Molecules. 2025 Feb 26;30(5):1058. doi: 10.3390/molecules30051058. Molecules. 2025. PMID: 40076282 Free PMC article. Review.
-
Update of Natural Products and Their Derivatives Targeting Epstein-Barr Infection.Viruses. 2024 Jan 15;16(1):124. doi: 10.3390/v16010124. Viruses. 2024. PMID: 38257824 Free PMC article. Review.
-
A study related to the treatment of gastric cancer with Xiang-Sha-Liu-Jun-Zi-Tang based on network analysis.Heliyon. 2023 Aug 28;9(9):e19546. doi: 10.1016/j.heliyon.2023.e19546. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809372 Free PMC article.
References
-
- Kuwajima H., Taneda Y., Chen W.Z., Kawanishi T., Hori K., Taniyama T., Kobayashi M., Ren J., Kitagawa I. Variation of chemical constituents in processed licorice roots: Quantitative determination of saponin and flavonoid constituents in bark removed and roasted licorice roots. Yakugaku Zasshi. 1999;119:945–955. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

