Rescue of Early bace-1 and Global DNA Demethylation by S-Adenosylmethionine Reduces Amyloid Pathology and Improves Cognition in an Alzheimer's Model
- PMID: 27681803
- PMCID: PMC5041108
- DOI: 10.1038/srep34051
Rescue of Early bace-1 and Global DNA Demethylation by S-Adenosylmethionine Reduces Amyloid Pathology and Improves Cognition in an Alzheimer's Model
Abstract
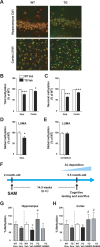
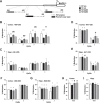
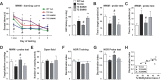
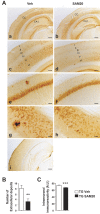
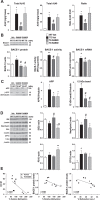
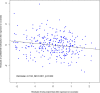
General DNA hypomethylation is associated with Alzheimer's disease (AD), but it is unclear when DNA hypomethylation starts or plays a role in AD pathology or whether DNA re-methylation would rescue early amyloid-related cognitive impairments. In an APP transgenic mouse model of AD-like amyloid pathology we found that early intraneuronal amyloid beta build-up is sufficient to unleash a global and beta-site amyloid precursor protein cleaving enzyme 1 (bace-1) DNA demethylation in AD-vulnerable brain regions. S-adenosylmethionine administration at these early stages abolished this hypomethylation, diminished the amyloid pathology and restored cognitive capabilities. To assess a possible human significance of findings, we examined the methylation at 12 CpGs sites in the bace-1 promoter, using genome-wide DNA methylation data from 740 postmortem human brains. Thus, we found significant associations of bace-1 promoter methylation with β-amyloid load among persons with AD dementia, and PHFtau tangle density. Our results support a plausible causal role for the earliest amyloid beta accumulation to provoke DNA hypomethylation, influencing AD pathological outcomes.
Figures
References
-
- Cuello A. C. Overview of the Alzheimer’s disease pathology and potential therapeutic targets. In Pharmacological mechanisms in Alzheimer’s therapeutics Cuello A. C., ed. (Springer, New York), pp. 1–27 (2007).
-
- Querfurth H. W. & LaFerla F. M. Alzheimer’s Disease. N Engl J Med 362, 329–344 (2010). - PubMed
-
- LaFerla F. M., Green K. N. & Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8, 499–509 (2007). - PubMed
-
- Han J. et al.. Effect of 5-aza-2-deoxycytidine microinjecting into hippocampus and prelimbic cortex on acquisition and retrieval of cocaine-induced place preference in C57BL/6 mice. Eur J Pharmacol 642, 93–98 (2010). - PubMed
-
- Levenson J. M. et al.. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem 281, 15763–15773 (2006). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

