Thermus thermophilus as a Source of Thermostable Lipolytic Enzymes
- PMID: 27682117
- PMCID: PMC5023265
- DOI: 10.3390/microorganisms3040792
Thermus thermophilus as a Source of Thermostable Lipolytic Enzymes
Abstract
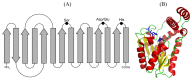
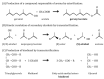
Lipolytic enzymes, esterases (EC 3.1.1.1) and lipases (EC 3.1.1.3), catalyze the hydrolysis of ester bonds between alcohols and carboxylic acids, and its formation in organic media. At present, they represent about 20% of commercialized enzymes for industrial use. Lipolytic enzymes from thermophilic microorganisms are preferred for industrial use to their mesophilic counterparts, mainly due to higher thermostability and resistance to several denaturing agents. However, the production at an industrial scale from the native organisms is technically complicated and expensive. The thermophilic bacterium Thermus thermophilus (T. thermophilus) has high levels of lipolytic activity, and its whole genome has been sequenced. One esterase from the T. thermophilus strain HB27 has been widely characterized, both in its native form and in recombinant forms, being expressed in mesophilic microorganisms. Other putative lipases/esterases annotated in the T. thermophilus genome have been explored and will also be reviewed in this paper.
Keywords: Thermus thermophilus; esterase; lipase; lipolytic; thermophilic.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

