Broad Detection of Alterations Predicted to Confer Lack of Benefit From EGFR Antibodies or Sensitivity to Targeted Therapy in Advanced Colorectal Cancer
- PMID: 27682134
- PMCID: PMC5189622
- DOI: 10.1634/theoncologist.2016-0148
Broad Detection of Alterations Predicted to Confer Lack of Benefit From EGFR Antibodies or Sensitivity to Targeted Therapy in Advanced Colorectal Cancer
Abstract
Introduction: A KRAS mutation represented the first genomic biomarker to predict lack of benefit from anti-epidermal growth factor receptor (EGFR) antibody therapy in advanced colorectal cancer (CRC). Expanded RAS testing has further refined the treatment approach, but understanding of genomic alterations underlying primary and acquired resistance is limited and further study is needed.
Materials and methods: We prospectively analyzed 4,422 clinical samples from patients with advanced CRC, using hybrid-capture based comprehensive genomic profiling (CGP) at the request of the individual treating physicians. Comparison with prior molecular testing results, when available, was performed to assess concordance.
Results: We identified a RAS/RAF pathway mutation or amplification in 62% of cases, including samples harboring KRAS mutations outside of the codon 12/13 hotspot region in 6.4% of cases. Among cases with KRAS non-codon 12/13 alterations for which prior test results were available, 79 of 90 (88%) were not identified by focused testing. Of 1,644 RAS/RAF wild-type cases analyzed by CGP, 31% harbored a genomic alteration (GA) associated with resistance to anti-EGFR therapy in advanced CRC including mutations in PIK3CA, PTEN, EGFR, and ERBB2. We also identified other targetable GA, including novel kinase fusions, receptor tyrosine kinase amplification, activating point mutations, as well as microsatellite instability.
Conclusion: Extended genomic profiling reliably detects alterations associated with lack of benefit to anti-EGFR therapy in advanced CRC, while simultaneously identifying alterations potentially important in guiding treatment. The use of CGP during the course of clinical care allows for the refined selection of appropriate targeted therapies and clinical trials, increasing the chance of clinical benefit and avoiding therapeutic futility.
Implications for practice: Comprehensive genomic profiling (CGP) detects diverse genomic alterations associated with lack of benefit to anti-epidermal growth factor receptor therapy in advanced colorectal cancer (CRC), as well as targetable alterations in many other genes. This includes detection of a broad spectrum of activating KRAS alterations frequently missed by focused molecular hotspot testing, as well as other RAS/RAF pathway alterations, mutations shown to disrupt antibody binding, RTK activating point mutations, amplifications, and rearrangements, and activating alterations in downstream effectors including PI3K and MEK1. The use of CGP in clinical practice is critical to guide appropriate selection of targeted therapies for patients with advanced CRC.
Keywords: Biomarker; Cetuximab; Colon cancer; Epidermal growth factor receptor; Genomic profiling; KRAS.
©AlphaMed Press.
Figures
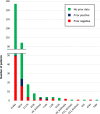
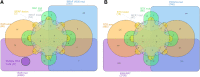
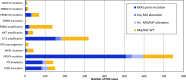
References
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. - PubMed
-
- Cremolini C, Loupakis F, Falcone A. FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2015;372:291–292. - PubMed
-
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

