Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models
- PMID: 27682618
- PMCID: PMC5149073
- DOI: 10.1161/CIRCRESAHA.116.309799
Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models
Abstract
Rationale: Vascular smooth muscle cell (VSMC) accumulation is a hallmark of atherosclerosis and vascular injury. However, fundamental aspects of proliferation and the phenotypic changes within individual VSMCs, which underlie vascular disease, remain unresolved. In particular, it is not known whether all VSMCs proliferate and display plasticity or whether individual cells can switch to multiple phenotypes.
Objective: To assess whether proliferation and plasticity in disease is a general characteristic of VSMCs or a feature of a subset of cells.
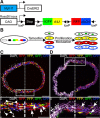
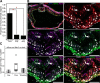
Methods and results: Using multicolor lineage labeling, we demonstrate that VSMCs in injury-induced neointimal lesions and in atherosclerotic plaques are oligoclonal, derived from few expanding cells. Lineage tracing also revealed that the progeny of individual VSMCs contributes to both alpha smooth muscle actin (aSma)-positive fibrous cap and Mac3-expressing macrophage-like plaque core cells. Costaining for phenotypic markers further identified a double-positive aSma+ Mac3+ cell population, which is specific to VSMC-derived plaque cells. In contrast, VSMC-derived cells generating the neointima after vascular injury generally retained the expression of VSMC markers and the upregulation of Mac3 was less pronounced. Monochromatic regions in atherosclerotic plaques and injury-induced neointima did not contain VSMC-derived cells expressing a different fluorescent reporter protein, suggesting that proliferation-independent VSMC migration does not make a major contribution to VSMC accumulation in vascular disease.
Conclusions: We demonstrate that extensive proliferation of a low proportion of highly plastic VSMCs results in the observed VSMC accumulation after injury and in atherosclerotic plaques. Therapeutic targeting of these hyperproliferating VSMCs might effectively reduce vascular disease without affecting vascular integrity.
Keywords: atherosclerosis; lineage-tracing; macrophages; neointima; phenotype; vascular diseases vascular smooth muscle.
© 2016 The Authors.
Conflict of interest statement
None
Figures





Comment in
-
Reconciling Smooth Muscle Cell Oligoclonality and Proliferative Capacity in Experimental Atherosclerosis.Circ Res. 2016 Dec 9;119(12):1262-1264. doi: 10.1161/CIRCRESAHA.116.310104. Circ Res. 2016. PMID: 27932466 Free PMC article. No abstract available.
References
-
- Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. - PubMed
-
- Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S, Weiser-Evans MC. SDF-1α induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300–1308. doi: 10.1161/ATVBAHA.111.223701. - PMC - PubMed
-
- Campbell JH, Campbell GR. Smooth muscle phenotypic modulation–a personal experience. Arterioscler Thromb Vasc Biol. 2012;32:1784–1789. doi: 10.1161/ATVBAHA.111.243212. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

