Interaction of microtubules with the actin cytoskeleton via cross-talk of EB1-containing +TIPs and γ-actin in epithelial cells
- PMID: 27683037
- PMCID: PMC5341938
- DOI: 10.18632/oncotarget.12236
Interaction of microtubules with the actin cytoskeleton via cross-talk of EB1-containing +TIPs and γ-actin in epithelial cells
Abstract
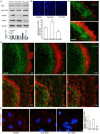
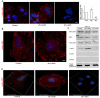
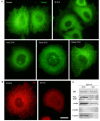
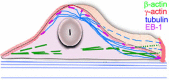
Actin microfilaments and microtubules are both highly dynamic cytoskeleton components implicated in a wide range of intracellular processes as well as cell-cell and cell-substrate interactions. The interactions of actin filaments with the microtubule system play an important role in the assembly and maintenance of 3D cell structure. Here we demonstrate that cytoplasmic actins are differentially distributed in relation to the microtubule system. LSM, 3D-SIM, proximity ligation assay (PLA) and co-immunoprecipitation methods applied in combination with selective depletion of β- or γ-cytoplasmic actins revealed a selective interaction between microtubules and γ-, but not β-cytoplasmic actin via the microtubule +TIPs protein EB1. EB1-positive comet distribution analysis and quantification have shown more effective microtubule growth in the absence of β-actin. Our data represent the first demonstration that microtubule +TIPs protein EB1 interacts mainly with γ-cytoplasmic actin in epithelial cells.
Keywords: +TIPs; EB1; actin isoforms; microtubules; β-actin; γ-actin.
Conflict of interest statement
Authors do not have any conflicts of interests.
Figures
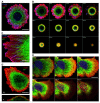
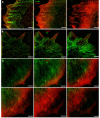
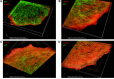
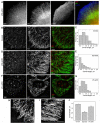

References
-
- Amann KJ, Pollard TD. Cellular regulation of actin network assembly. Curr. Biol. 2000;10:728–730. - PubMed
-
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984a;312:237–242. - PubMed
-
- Mitchison T, Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984b;312:232–237. - PubMed
-
- Vorobjev I A, Rodionov V I, Maly I V, Borisy GG. Contribution of plus and minus end pathways to microtubule turnover. J Cell Sci. 1999;112:2277–2289. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

