Pseudoexons provide a mechanism for allele-specific expression of APC in familial adenomatous polyposis
- PMID: 27683109
- PMCID: PMC5342583
- DOI: 10.18632/oncotarget.12206
Pseudoexons provide a mechanism for allele-specific expression of APC in familial adenomatous polyposis
Abstract
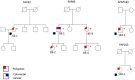
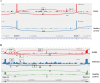
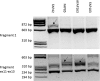
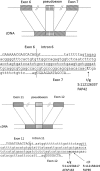
Allele-specific expression (ASE) of the Adenomatous Polyposis Coli (APC) gene occurs in up to one-third of families with adenomatous polyposis (FAP) that have screened mutation-negative by conventional techniques. To advance our understanding of the genomic basis of this phenomenon, 54 APC mutation-negative families (21 with classical FAP and 33 with attenuated FAP, AFAP) were investigated. We focused on four families with validated ASE and scrutinized these families by sequencing of the blood transcriptomes (RNA-seq) and genomes (WGS). Three families, two with classical FAP and one with AFAP, revealed deep intronic mutations associated with pseudoexons. In all three families, intronic mutations (c.646-1806T>G in intron 6, c.1408+729A>G in intron 11, and c.1408+731C>T in intron 11) created new splice donor sites resulting in the insertion of intronic sequences (of 127 bp, 83 bp, and 83 bp, respectively) in the APC transcript. The respective intronic mutations were absent in the remaining polyposis families and the general population. Premature stop of translation as the predicted consequence as well as co-segregation with polyposis supported the pathogenicity of the pseudoexons. We conclude that next generation sequencing on RNA and genomic DNA is an effective strategy to reveal and validate pseudoexons that are regularly missed by traditional screening methods and is worth considering in apparent mutation-negative polyposis families.
Keywords: APC; RNA-seq; allele-specific expression; familial adenomatous polyposis; pseudoexon.
Conflict of interest statement
No conflicts of interests.
Figures

References
-
- Aretz S, Stienen D, Friedrichs N, Stemmler S, Uhlhaas S, Rahner N, Propping P, Friedl W. Somatic APC mosaicism: a frequent cause of familial adenomatous polyposis (FAP) Hum Mutat. 2007;28:985–992. - PubMed
-
- Hegde M, Ferber M, Mao R, Samowitz W, Ganguly A, Working Group of the American College of Medical Genetics. Genomics (ACMG) Laboratory Quality Assurance Committee ACMG technical standards and guidelines for genetic testing for inherited colorectal cancer (Lynch syndrome, familial adenomatous polyposis, and MYH-associated polyposis) Genet Med. 2014;16:101–116. - PubMed
-
- Necker J, Kovac M, Attenhofer M, Reichlin B, Heinimann K. Detection of APC germ line mosaicism in patients with de novo familial adenomatous polyposis: a plea for the protein truncation test. J Med Genet. 2011;48:526–529. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

