Metastasis and Circulating Tumor Cells
- PMID: 27683421
- PMCID: PMC4975257
Metastasis and Circulating Tumor Cells
Abstract
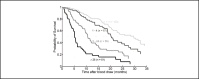
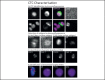
Cancer is a prominent cause of death worldwide. In most cases, it is not the primary tumor which causes death, but the metastases. Metastatic tumors are spread over the entire human body and are more difficult to remove or treat than the primary tumor. In a patient with metastatic disease, circulating tumor cells (CTCs) can be found in venous blood. These circulating tumor cells are part of the metastatic cascade. Clinical studies have shown that these cells can be used to predict treatment response and their presence is strongly associated with poor survival prospects. Enumeration and characterization of CTCs is important as this can help clinicians make more informed decisions when choosing or evaluating treatment. CTC counts are being included in an increasing number of studies and thus are becoming a bigger part of disease diagnosis and therapy management. We present an overview of the most prominent CTC enumeration and characterization methods and discuss the assumptions made about the CTC phenotype. Extensive CTC characterization of for example the DNA, RNA and antigen expression may lead to more understanding of the metastatic process.
Conflict of interest statement
Conflict of interest: LWMM is consultant for Veridex LLC, Raritan, NJ
Figures




References
-
- Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D., Global cancer statistics. CA: a cancer journal for clinicians, 2011. 61(2): p. 69-90. - PubMed
-
- Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C., Reuben J.M., Doyle G.V., Allard W.J., Terstappen L.W.M.M., Hayes D.F., Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New England Journal of Medicine, 2004. 351(8): p. 781-791. - PubMed
-
- Cohen S.J., Punt C.J.A., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C., Doyle G.V., Tissing H., Terstappen L.W.M.M., Meropol N.J., Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients With Metastatic Colorectal Cancer. Journal of Clinical Oncology, 2008. 26(19): p. 3213-3221. - PubMed
-
- de Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W.W.M., Pienta K.J., Raghavan D., Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clinical Cancer Research, 2008. 14(19): p. 6302-6309. - PubMed
LinkOut - more resources
Full Text Sources
