Novel signaling pathways in pulmonary arterial hypertension (2015 Grover Conference Series)
- PMID: 27683605
- PMCID: PMC5019081
- DOI: 10.1086/688034
Novel signaling pathways in pulmonary arterial hypertension (2015 Grover Conference Series)
Erratum in
-
Corrigendum.Pulm Circ. 2017 Apr-Jun;7(2):559. doi: 10.1177/2045893217706334. Pulm Circ. 2017. PMID: 28597768 Free PMC article. No abstract available.
Abstract
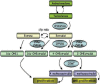
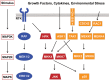
The proliferative endothelial and smooth muscle cell phenotype, inflammation, and pulmonary vascular remodeling are prominent features of pulmonary arterial hypertension (PAH). Mutations in bone morphogenetic protein type 2 receptor (BMPR2) have been identified as the most common genetic cause of PAH and females with BMPR2 mutations are 2.5 times as likely to develop heritable forms of PAH than males. Higher levels of estrogen have also been observed in males with PAH, implicating sex hormones in PAH pathogenesis. Recently, the estrogen metabolite 16α-OHE1 (hydroxyestrone) was implicated in the regulation of miR29, a microRNA involved in modulating energy metabolism. In females, decreased miR96 enhances serotonin's effect by upregulating the 5-hydroxytryptamine 1B (5HT1B) receptor. Because PAH is characterized as a quasi-malignant disease, likely due to BMPR2 loss of function, altered signaling pathways that sustain this cancer-like phenotype are being explored. Extracellular signal-regulated kinases 1 and 2 and p38 mitogen-activated protein kinases (MAPKs) play a critical role in proliferation and cell motility, and dysregulated MAPK signaling is observed in various experimental models of PAH. Wnt signaling pathways preserve pulmonary vascular homeostasis, and dysregulation of this pathway could contribute to limited vascular regeneration in response to injury. In this review, we take a closer look at sex, sex hormones, and the interplay between sex hormones and microRNA regulation. We also focus on MAPK and Wnt signaling pathways in the emergence of a proproliferative, antiapoptotic endothelial phenotype, which then orchestrates an angioproliferative process of vascular remodeling, with the hope of developing novel therapies that could reverse the phenotype.
Keywords: Wnt; microRNA; mitogen-activated protein kinase; sex hormones; vascular remodeling.
Figures


References
-
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43(12 suppl):S13–S24. - PubMed
-
- Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers: evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 1999;155(2):411–419. - PMC - PubMed
-
- Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 2001;195(3):367–374. - PubMed
-
- Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock 2008;30(6):660–667. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

