The Neurotrophic Factor Receptor p75 in the Rat Dorsolateral Striatum Drives Excessive Alcohol Drinking
- PMID: 27683907
- PMCID: PMC5039257
- DOI: 10.1523/JNEUROSCI.4597-14.2016
The Neurotrophic Factor Receptor p75 in the Rat Dorsolateral Striatum Drives Excessive Alcohol Drinking
Abstract
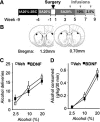
Brain-derived neurotrophic factor (BDNF) signaling in the dorsolateral striatum (DLS) keeps alcohol intake in moderation. For example, activation of the BDNF receptor tropomyosin receptor kinase B (TrkB) in the DLS reduces intake in rats that consume moderate amounts of alcohol. Here, we tested whether long-term excessive consumption of alcohol produces neuroadaptations in BDNF signaling in the rat DLS. We found that BDNF was no longer able to gate alcohol self-administration after a history of repeated cycles of binge alcohol drinking and withdrawal. We then elucidated the possible neuroadaptations that could block the ability of BDNF to keep consumption of alcohol in moderation. We report that intermittent access to 20% alcohol in a two-bottle choice paradigm that models excessive alcohol drinking produces a mobilization of DLS p75 neurotrophin receptor (p75NTR), whose activities oppose those of the Trk receptors, including TrkB. These neuroadaptations were not observed in the DLS of rats exposed to continuous access to 10% alcohol or in rats consuming sucrose. Furthermore, short hairpin RNA (shRNA)-mediated knockdown of the p75NTR gene in the DLS, as well as intra-DLS infusion or systemic administration of the p75NTR modulator, LM11A-31, significantly reduced binge drinking of alcohol. Together, our results suggest that excessive alcohol consumption produces a change in BDNF signaling in the DLS, which is mediated by the recruitment of p75NTR. Our data also imply that modulators of p75NTR signaling could be developed as medications for alcohol abuse disorders.
Significance statement: Neuroadaptations gate or drive excessive, compulsive alcohol drinking. We previously showed that brain-derived neurotrophic factor and its receptor, TrkB, in the dorsolateral striatum (DLS), are part of an endogenous system that keeps alcohol drinking in moderation. Here, we show that a history of excessive alcohol intake produces neuroadaptations in the DLS that preclude BDNF's ability to gate alcohol self-administration in rats by the recruitment of the low-affinity neurotrophin receptor, p75NTR, whose activities opposes those of the Trk receptors. Finally, we show that the administration of the p75NTR modulator, LM11A-31, significantly reduces excessive alcohol intake suggesting that the drug may be developed as a new treatment for alcohol abuse disorders.
Keywords: BDNF; addiction; alcohol; dorsal striatum; neurotrophic factor; p75NTR.
Copyright © 2016 the authors 0270-6474/16/3610116-12$15.00/0.
Figures






References
-
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology. 2014;39:2835–2845. doi: 10.1038/npp.2014.135. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
