Chemogenetic Activation of an Extinction Neural Circuit Reduces Cue-Induced Reinstatement of Cocaine Seeking
- PMID: 27683912
- PMCID: PMC5039261
- DOI: 10.1523/JNEUROSCI.0773-16.2016
Chemogenetic Activation of an Extinction Neural Circuit Reduces Cue-Induced Reinstatement of Cocaine Seeking
Abstract
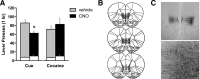
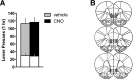
The ventromedial prefrontal cortex (vmPFC) has been shown to negatively regulate cocaine-seeking behavior, but the precise conditions by which vmPFC activity can be exploited to reduce cocaine relapse are currently unknown. We used viral-mediated gene transfer of designer receptors (DREADDs) to activate vmPFC neurons and examine the consequences on cocaine seeking in a rat self-administration model of relapse. Activation of vmPFC neurons with the Gq-DREADD reduced reinstatement of cocaine seeking elicited by cocaine-associated cues, but not by cocaine itself. We used a retro-DREADD approach to confine the Gq-DREADD to vmPFC neurons that project to the medial nucleus accumbens shell, confirming that these neurons are responsible for the decreased cue-induced reinstatement of cocaine seeking. The effects of vmPFC activation on cue-induced reinstatement depended on prior extinction training, consistent with the reported role of this structure in extinction memory. These data help define the conditions under which chemogenetic activation of extinction neural circuits can be exploited to reduce relapse triggered by reminder cues.
Significance statement: The ventromedial prefrontal cortex (vmPFC) projection to the nucleus accumbens shell is important for extinction of cocaine seeking, but its anatomical proximity to the relapse-promoting projection from the dorsomedial prefrontal cortex to the nucleus accumbens core makes it difficult to selectively enhance neuronal activity in one pathway or the other using traditional pharmacotherapy (e.g., systemically administered drugs). Viral-mediated gene delivery of an activating Gq-DREADD to vmPFC and/or vmPFC projections to the nucleus accumbens shell allows the chemogenetic exploitation of this extinction neural circuit to reduce cocaine seeking and was particularly effective against relapse triggered by cocaine reminder cues.
Keywords: DREADD; extinction; infralimbic; memory; nucleus accumbens shell.
Copyright © 2016 the authors 0270-6474/16/3610174-07$15.00/0.
Figures

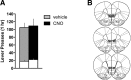
References
-
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
