Pregabalin for pain in fibromyalgia in adults
- PMID: 27684492
- PMCID: PMC6457745
- DOI: 10.1002/14651858.CD011790.pub2
Pregabalin for pain in fibromyalgia in adults
Abstract
Background: This review updates part of an earlier Cochrane review on 'Pregabalin for acute and chronic pain in adults' (Moore 2009), and considers only fibromyalgia pain.Antiepileptic drugs have been used in pain management since the 1960s. Pregabalin is an antiepileptic drug also used in management of chronic pain conditions, including fibromyalgia. Pain response with pregabalin is associated with major benefits for other symptoms, and improved quality of life and function in people with chronic painful conditions.
Objectives: To assess the analgesic efficacy and adverse events of pregabalin for pain in fibromyalgia in adults, compared with placebo or any active comparator.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and EMBASE for randomised controlled trials from inception to May 2009 for the original review and to 16 March 2016 for this update. We also searched the reference lists of retrieved studies and reviews, and online clinical trial registries.
Selection criteria: We included randomised, double-blind trials of eight weeks' duration or longer, comparing pregabalin with placebo or another active treatment for relief of pain in fibromyalgia, and reporting on the analgesic effect of pregabalin, with subjective pain assessment by the participant.
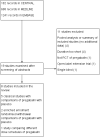
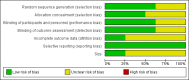
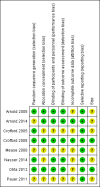
Data collection and analysis: Two review authors independently extracted data and assessed trial quality and potential bias. Primary outcomes were participants with moderate pain relief (at least 30% pain relief over baseline or much or very much improved on Patient Global Impression of Change scale (PGIC)) or substantial pain relief (at least 50% pain relief over baseline or very much improved on PGIC). Where pooled analysis was possible, we used dichotomous data to calculate risk ratio and number needed to treat (NNT), using standard methods. We assessed the quality of the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created 'Summary of findings' tables.
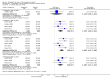
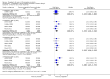
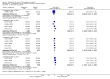
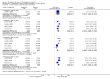
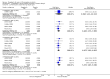
Main results: Our searches identified two new published studies with classic design, and one new published study with an enriched enrolment randomised withdrawal (EERW) design.We included eight studies. Five (3283 participants) had a classic design in which participants were randomised at the start of the study to pregabalin (150, 300, 450, or 600 mg daily) or placebo, with assessment after 8 to 13 weeks of stable treatment. No studies included active comparators. Studies had low risk of bias, except that the last observation carried forward (LOCF) imputation method used in analyses of the primary outcomes could overestimate treatment effect.Pregabalin increased the number of participants experiencing substantial benefit (at least 50% pain intensity reduction after 12 or 13 weeks' stable treatment (450 mg: RR 1.8, 95% CI 1.4 to 2.1, 1874 participants, 5 studies, high quality evidence)). Substantial benefit with pregabalin 300 to 600 mg was experienced by about 14% of participants with placebo, but about 9% more with pregabalin 300 to 600 mg (22% to 24%) (high quality evidence). Pregabalin increased the number of participants experiencing moderate benefit (at least 30% pain intensity reduction after 12 or 13 weeks' stable treatment) (450 mg: RR 1.5, 95% CI (1.3 to 1.7), 1874 participants, 5 studies, high quality evidence). Moderate benefit with pregabalin 300 to 600 mg was experienced by about 28% of participants with placebo, but about 11% more with pregabalin 300 to 600 mg (39% to 43%) (high quality evidence). A similar magnitude of effect was found using PGIC of 'very much improved' and 'much or very much improved'. NNTs for these outcomes ranged between 7 and 14 (high quality evidence).A small study (177 participants) compared nightly with twice-daily pregabalin, and concluded there was no difference in effect.Two studies (1492 participants began initial dose titration, 687 participants randomised) had an EERW design in which those with good pain relief after titration were randomised, double blind, to continuing the effective dose (300 to 600 mg pregabalin daily) or a short down-titration to placebo for 13 or 26 weeks. We calculated the outcome of maintained therapeutic response (MTR) without withdrawal, equivalent to a moderate benefit. Of those randomised, 40% had MTR with pregabalin and 20% with placebo (high quality evidence). The NNT was 5, but normalised to the starting population tested it was 12. About 10% of the initial population would have achieved the MTR outcome, similar to the result from studies of classic design. MTR had no imputation concerns.The majority (70% to 90%) of participants in all treatment groups experienced adverse events. Specific adverse events were more common with pregabalin than placebo, in particular dizziness, somnolence, weight gain, and peripheral oedema, with number needed to harm of 3.7, 7.4, 18, and 19 respectively for all doses combined (high quality evidence). Serious adverse events did not differ between active treatment groups and placebo (very low quality evidence). Withdrawals for any reason were more common with pregabalin than placebo only with the 600 mg dose in studies of classic design. Withdrawals due to adverse events were about 10% higher with pregabalin than placebo, but withdrawals due to lack of efficacy were about 6% lower (high quality evidence).
Authors' conclusions: Pregabalin 300 to 600 mg produces a major reduction in pain intensity over 12 to 26 weeks with tolerable adverse events for a small proportion of people (about 10% more than placebo) with moderate or severe pain due to fibromyalgia. The degree of pain relief is known to be accompanied by improvements in other symptoms, quality of life, and function. These results are similar to other effective medicines in fibromyalgia (milnacipran, duloxetine).
Conflict of interest statement
SD: none known.
MC: none known.
PW: none known.
SL: none known; SL is a specialist pain physician and manages patients with fibromyalgia.
TP: none known; TP is a specialist pain physician and manages patients with fibromyalgia.
RAM has received grant support from RB relating to individual patient level analyses of trial data on ibuprofen in acute pain and the effects of food on drug absorption of analgesics (2013), and from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015).
Figures












Comment in
-
Review: Pregabalin reduces fibromyalgia pain but increases adverse events.Ann Intern Med. 2017 Jan 17;166(2):JC2. doi: 10.7326/ACPJC-2017-166-2-002. Ann Intern Med. 2017. PMID: 28114458 No abstract available.
-
Pregabalin is effective in reducing fibromyalgia pain.Evid Based Med. 2017 Apr;22(2):70-71. doi: 10.1136/ebmed-2016-110630. Epub 2017 Feb 27. Evid Based Med. 2017. PMID: 28242609 No abstract available.
References
References to studies included in this review
Arnold 2008 {published data only}
Arnold 2014 {published data only}
-
- Arnold LM, Arsenault P, Huffman C, Patrick JL, Messig M, Chew ML, et al. Once daily controlled‐release pregabalin in the treatment of patients with fibromyalgia: a phase III, double‐blind, randomized withdrawal, placebo‐controlled study. Current Medical Research and Opinion 2014;30(10):2069‐83. [CTG: NCT01271933; DOI: 10.1185/03007995.2014.928275] - DOI - PubMed
Crofford 2005 {published data only}
-
- Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Pregabalin 1008‐105 Study Group, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2005;52(4):1264‐73. [DOI: 10.1002/art.20983] - DOI - PubMed
Crofford 2008 {published data only}
-
- Crofford LJ, Mease PJ, Simpson SL, Young JP Jr, Martin SA, Haig GM, et al. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6‐month, double‐blind, placebo‐controlled trial with pregabalin. Pain 2008;136(3):419‐31. [DOI: 10.1016/j.pain.2008.02.027] - DOI - PubMed
Mease 2008 {published data only}
-
- Mease PJ, Russell IJ, Arnold LM, Florian H, Young JP Jr, Martin SA, et al. A randomized, double‐blind, placebo‐controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. Journal of Rheumatology 2008;35(3):502‐14. [CTG: NCT00645398] - PubMed
Nasser 2014 {published data only}
Ohta 2012 {published data only}
-
- Ohta H, Oka H, Usui C, Ohkura M, Suzuki M, Nishioka K. A randomized, double‐blind, multicenter, placebo‐controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia. Arthritis Research and Therapy 2012;14(5):R217. [CTG: NCT00830167; DOI: 10.1186/ar4056] - DOI - PMC - PubMed
Pauer 2011 {published data only}
-
- A 14‐week, randomized, double‐blind, placebo‐controlled trial of pregabalin twice daily in patients with fibromyalgia. PhRMA Web Synopsis 7 March 2008.
-
- Pauer L, Winkelmann A, Arsenault P, Jespersen A, Whelan L, Atkinson G, et al. An international, randomized, double‐blind, placebo‐controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgia. Journal of Rheumatology 2011;38(12):2643‐52. [CTG: NCT00333866; DOI: 10.3899/jrheum.110569] - DOI - PubMed
References to studies excluded from this review
Arnold 2012 {published data only}
Arnold 2015 {published data only}
-
- Arnold LM, Sarzi‐Puttini P, Arsenault P, Khan T, Bhadra Brown P, Clair A, et al. Efficacy and safety of pregabalin in patients with fibromyalgia and comorbid depression taking concurrent antidepressant medication: a randomized, placebo‐controlled study. Journal of Rheumatology 2015;42(7):1237‐44. [DOI: 10.3899/jrheum.141196] - DOI - PubMed
-
- NCT01432236. A phase 3b multicenter, double‐blind, randomized, placebo‐controlled, 2‐way crossover study of pregabalin in the treatment of fibromyalgia with concurrent antidepressant therapy for comorbid depression. clinicaltrials.gov/ct2/show/NCT01432236 (accessed 16 March 2016).
Byon 2010 {published data only}
-
- Byon W, Ouellet D, Chew M, Ito K, Burger P, Pauer L, et al. Exposure‐response analyses of the effects of pregabalin in patients with fibromyalgia using daily pain scores and patient global impression of change. Journal of Clinical Pharmacology 2010;50(7):803‐15. [DOI: 10.1177/0091270009352187] - DOI - PubMed
Emir 2010 {published data only}
NCT00760474 {unpublished data only}
-
- NCT00760474. A double‐blind, placebo‐controlled cross‐over study in fibromyalgia subjects to examine effects of pregabalin on brain response to mechanical pain as assessed by functional magnetic resonance imaging, proton magnetic resonance spectroscopy and subjective ratings. clinicaltrials.gov/ct2/show/NCT00760474 (accessed 16 March 2016).
NCT01268631 {unpublished data only}
-
- NCT01268631. Mechanism‐based choice of therapy: can treatments success in fibromyalgia patients be coupled to psychophysical pain modulation profile?. clinicaltrials.gov/ct2/show/NCT01268631 (accessed 16 March 2016).
NCT01904097 {unpublished data only}
-
- NCT01904097. Study of the brain with optic functional neuroimaging in patients with chronic pain using transcranial direct current stimulation. clinicaltrials.gov/ct2/show/NCT01904097 (accessed 16 March 2016).
Ohta 2013 {published data only}
-
- Ohta H, Oka H, Usui C, Ohkura M, Suzuki M, Nishioka K. An open‐label long‐term phase III extension trial to evaluate the safety and efficacy of pregabalin in Japanese patients with fibromyalgia. Modern Rheumatology/the Japan Rheumatism Association 2013;23(6):1108‐15. [DOI: 10.1007/s10165-012-0803-x] - DOI - PubMed
Ramzy 2016 {published data only}
Roth 2012 {published data only}
-
- Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized, placebo‐controlled, 2‐way crossover polysomnography study. Arthritis Care and Research 2012;64(4):597‐606. [DOI: 10.1002/acr.21595] - DOI - PubMed
References to ongoing studies
NCT01387607 {unpublished data only}
-
- NCT01387607. A 14‐week, randomized, double‐blind placebo‐controlled study for pregabalin in patients with fibromyalgia. clinicaltrials.gov/ct2/show/NCT01387607 (accessed 16 March 2016). [A0081241]
NCT02146430 {unpublished data only}
-
- NCT02146430. A randomized, double‐blind, placebo‐ and active‐controlled study of DS‐5565 for treatment of pain associated with fibromyalgia. clinicaltrials.gov/ct2/show/NCT02146430 (accessed 16 March 2016). [Sponsor ID: DS5565‐A‐E309]
NCT02187159 {unpublished data only}
-
- NCT02187159. A randomized, double‐blind, placebo‐ and active‐controlled study of DS‐5565 for treatment of pain associated with fibromyalgia. clinicaltrials.gov/ct2/show/NCT02187159 (accessed 16 March 2016). [Sponsor ID: DS5565‐A‐E311]
Additional references
Arnold 2013
Bennett 2007
Bockbrader 2010
Bradley 2009
Choi 2010
Clauw 2014
Collins 1997
Conaghan 2015
-
- Conaghan PG, Peloso PM, Everett SV, Rajagopalan S, Black CM, Mavros P, et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real‐world therapies. Rheumatology (Oxford) 2015;54(2):270‐7. [DOI: 10.1093/rheumatology/keu332] - DOI - PMC - PubMed
Cording 2015
Dechartres 2013
Dechartres 2014
Dworkin 2008
Eich 2012
EMC 2016
-
- Pregabalin. Electronic Medicines Compendium. www.medicines.org.uk/emc/medicine/14651 (accessed 16 March 2016).
Fitzcharles 2013
Forseth 1999
-
- Forseth KO, Husby G, Gran JT, Førre O. Prognostic factors for the development of fibromyalgia in women with self‐reported musculoskeletal pain. A prospective study. Journal of Rheumatology 1999;26:2458‐67. [PUBMED: 10555910] - PubMed
Guyatt 2011
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] - DOI - PubMed
Guyatt 2013b
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available at www.cochrane‐handbook.org.
Hoffman 2010
Häuser 2008
Häuser 2010
Häuser 2011
Häuser 2013a
-
- Häuser W, Galek A, Erbslöh‐Möller B, Köllner V, Kühn‐Becker H, Langhorst J, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013;154:1216‐23. [DOI: 10.1016/j.pain.2013.03.034] - DOI - PubMed
Häuser 2013b
Häuser 2014
Häuser 2015
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] - PubMed
Kalso 2013
Kim 2013
Koroschetz 2011
L'Abbé 1987
Lange 2010
Lee 2012
Lunn 2014
Macfarlane 2016
Mansfield 2016
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐262718‐X]
McQuay 2008
Mills 2015
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5]
Moore 2010a
Moore 2010b
Moore 2010c
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010d
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] - DOI - PubMed
Moore 2011b
Moore 2012a
Moore 2012b
Moore 2013a
Moore 2013b
Moore 2014a
Moore 2014b
Moore 2014c
Moore 2015
Mork 2010
O'Brien 2010
Oaklander 2013
PaPaS 2012
-
- PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 16 March 2016).
Queiroz 2013
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sommer 2012
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010a
Straube 2010b
Straube 2011
Sultan 2008
Taylor 2007
Tzellos 2010
-
- Tzellos TG, Toulis KA, Goulis DG, Papazisis G, Zampeli VA, Vakfari A, et al. Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta‐analysis. Journal of Clinical Pharmacology and Therapeutics 2010;35(6):239‐56. [DOI: 10.1111/j.1365-2710.2009.01144.x] - DOI - PubMed
Vos 2012
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
Walitt 2015
Wiffen 2013
Wolfe 1990
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33(2):160‐72. [DOI: 10.1002/art.1780330203] - DOI - PubMed
Wolfe 2010
Wolfe 2011
-
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. Journal of Rheumatology 2011;38:1113‐22. [DOI: 10.3899/jrheum.100594] - DOI - PubMed
Wolfe 2012
Wolfe 2013
Wolfe 2014
Yunus 2008
Üçeyler 2013a
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

