Inactivation of Prions and Amyloid Seeds with Hypochlorous Acid
- PMID: 27685252
- PMCID: PMC5042475
- DOI: 10.1371/journal.ppat.1005914
Inactivation of Prions and Amyloid Seeds with Hypochlorous Acid
Abstract
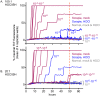
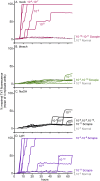
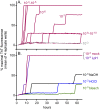
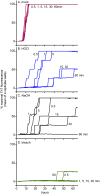
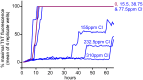
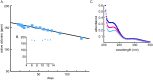
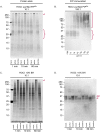
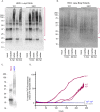
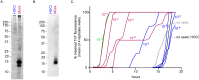
Hypochlorous acid (HOCl) is produced naturally by neutrophils and other cells to kill conventional microbes in vivo. Synthetic preparations containing HOCl can also be effective as microbial disinfectants. Here we have tested whether HOCl can also inactivate prions and other self-propagating protein amyloid seeds. Prions are deadly pathogens that are notoriously difficult to inactivate, and standard microbial disinfection protocols are often inadequate. Recommended treatments for prion decontamination include strongly basic (pH ≥~12) sodium hypochlorite bleach, ≥1 N sodium hydroxide, and/or prolonged autoclaving. These treatments are damaging and/or unsuitable for many clinical, agricultural and environmental applications. We have tested the anti-prion activity of a weakly acidic aqueous formulation of HOCl (BrioHOCl) that poses no apparent hazard to either users or many surfaces. For example, BrioHOCl can be applied directly to skin and mucous membranes and has been aerosolized to treat entire rooms without apparent deleterious effects. Here, we demonstrate that immersion in BrioHOCl can inactivate not only a range of target microbes, including spores of Bacillus subtilis, but also prions in tissue suspensions and on stainless steel. Real-time quaking-induced conversion (RT-QuIC) assays showed that BrioHOCl treatments eliminated all detectable prion seeding activity of human Creutzfeldt-Jakob disease, bovine spongiform encephalopathy, cervine chronic wasting disease, sheep scrapie and hamster scrapie; these findings indicated reductions of ≥103- to 106-fold. Transgenic mouse bioassays showed that all detectable hamster-adapted scrapie infectivity in brain homogenates or on steel wires was eliminated, representing reductions of ≥~105.75-fold and >104-fold, respectively. Inactivation of RT-QuIC seeding activity correlated with free chlorine concentration and higher order aggregation or destruction of proteins generally, including prion protein. BrioHOCl treatments had similar effects on amyloids composed of human α-synuclein and a fragment of human tau. These results indicate that HOCl can block the self-propagating activity of prions and other amyloids.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: DT is founder and CEO of BrioTech Inc, which sells BrioHOClTM. A gift from BrioTech was used to support the work at UW Bothell lead by LR. JFW is Chief Scientific Officer, corporate executive and shareholder of Briotech Inc.
Figures

References
-
- Parchi P, de BL, Saverioni D, Cohen ML, Ferrer I, Gambetti P, et al. Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol. 2012;124(4):517–29. 10.1007/s00401-012-1002-8 - DOI - PMC - PubMed
-
- Zanusso G, Monaco S. Bovine Spongiform Encephalopathy In: Zou W, Gambetti P, editors. Prions and Diseases. 2 New York: Springer; 2013. p. 1–14.
-
- Fast C, Groschup M. Classical and Atypical Scrapie in Sheep and Goats In: Zou W, Gambetti P, editors. Prions and Diseases. 2 New York: Springer; 2013. p. 15–44.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

