Lateral opening in the intact β-barrel assembly machinery captured by cryo-EM
- PMID: 27686148
- PMCID: PMC5056442
- DOI: 10.1038/ncomms12865
Lateral opening in the intact β-barrel assembly machinery captured by cryo-EM
Abstract
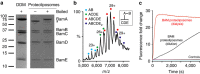
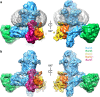
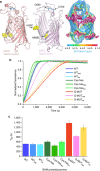
The β-barrel assembly machinery (BAM) is a ∼203 kDa complex of five proteins (BamA-E), which is essential for viability in E. coli. BAM promotes the folding and insertion of β-barrel proteins into the outer membrane via a poorly understood mechanism. Several current models suggest that BAM functions through a 'lateral gating' motion of the β-barrel of BamA. Here we present a cryo-EM structure of the BamABCDE complex, at 4.9 Å resolution. The structure is in a laterally open conformation showing that gating is independent of BamB binding. We describe conformational changes throughout the complex and interactions between BamA, B, D and E, and the detergent micelle that suggest communication between BAM and the lipid bilayer. Finally, using an enhanced reconstitution protocol and functional assays, we show that for the outer membrane protein OmpT, efficient folding in vitro requires lateral gating in BAM.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

