Ultraviolet-B Radiation (UV-B) Relieves Chilling-Light-Induced PSI Photoinhibition And Accelerates The Recovery Of CO2 Assimilation In Cucumber (Cucumis sativus L.) Leaves
- PMID: 27686324
- PMCID: PMC5043378
- DOI: 10.1038/srep34455
Ultraviolet-B Radiation (UV-B) Relieves Chilling-Light-Induced PSI Photoinhibition And Accelerates The Recovery Of CO2 Assimilation In Cucumber (Cucumis sativus L.) Leaves
Abstract
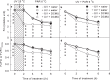
Ultraviolet-B radiation (UV-B) is generally considered to negatively impact the photosynthetic apparatus and plant growth. UV-B damages PSII but does not directly influence PSI. However, PSI and PSII successively drive photosynthetic electron transfer, therefore, the interaction between these systems is unavoidable. So we speculated that UV-B could indirectly affect PSI under chilling-light conditions. To test this hypothesis, the cucumber leaves were illuminated by UV-B prior or during the chilling-light treatment, and the leaves were then transferred to 25 °C and low-light conditions for recovery. The results showed that UV-B decreased the electron transfer to PSI by inactivating the oxygen-evolving complex (OEC), thereby protecting PSI from chilling-light-induced photoinhibition. This effect advantages the recoveries of PSI and CO2 assimilation after chilling-light stress, therefore should minimize the yield loss caused by chilling-light stress. Because sunlight consists of both UV-B and visible light, we suggest that UV-B-induced OEC inactivation is critical for chilling-light-induced PSI photoinhibition in field. Moreover, additional UV-B irradiation is an effective strategy to relieve PSI photoinhibition and yield loss in protected cultivation during winter. This study also demonstrates that minimizing the photoinhibition of PSI rather than that of PSII is essential for the chilling-light tolerance of the plant photosynthetic apparatus.
Figures






References
-
- Jansen M. A., Gaba V. & Greenberg B. M. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 3, 131–135 (1998).
-
- Kataria S., Jajoo A. & Guruprasad K. N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photoch. Photobio. B 137, 55–66 (2014). - PubMed
-
- Zhao D., Reddy K. R., Kakani V. G., Read J. J. & Sullivan J. H. Growth and physiological responses of cotton (Gossypium hirsutum L.) to elevated carbon dioxide and ultraviolet-B radiation under controlp environmental conditions. Plant Cell Environ. 26, 771–782 (2003).
LinkOut - more resources
Full Text Sources
Other Literature Sources

