Risk of thrombosis according to need of phlebotomies in patients with polycythemia vera treated with hydroxyurea
- PMID: 27686377
- PMCID: PMC5210240
- DOI: 10.3324/haematol.2016.152769
Risk of thrombosis according to need of phlebotomies in patients with polycythemia vera treated with hydroxyurea
Abstract
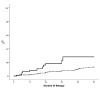
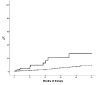
Hematocrit control below 45% is associated with a lower rate of thrombosis in polycythemia vera. In patients receiving hydroxyurea, this target can be achieved with hydroxyurea alone or with the combination of hydroxyurea plus phlebotomies. However, the clinical implications of phlebotomy requirement under hydroxyurea therapy are unknown. The aim of this study was to evaluate the need for additional phlebotomies during the first five years of hydroxyurea therapy in 533 patients with polycythemia vera. Patients requiring 3 or more phlebotomies per year (n=85, 16%) showed a worse hematocrit control than those requiring 2 or less phlebotomies per year (n=448, 84%). There were no significant differences between the two study groups regarding leukocyte and platelet counts. Patients requiring 3 or more phlebotomies per year received significantly higher doses of hydroxyurea than the remaining patients. A significant higher rate of thrombosis was found in patients treated with hydroxyurea plus 3 or more phlebotomies per year compared to hydroxyurea with 0-2 phlebotomies per year (20.5% vs. 5.3% at 3 years; P<0.0001). In multivariate analysis, independent risk factors for thrombosis were phlebotomy dependency (HR: 3.3, 95%CI: 1.5-6.9; P=0.002) and thrombosis at diagnosis (HR: 4.7, 95%CI: 2.3-9.8; P<0.0001). The proportion of patients fulfilling the European LeukemiaNet criteria of resistance/intolerance to hydroxyurea was significantly higher in the group requiring 3 or more phlebotomies per year (18.7% vs. 7.1%; P=0.001) mainly due to extrahematologic toxicity. In conclusion, phlebotomy requirement under hydroxyurea therapy identifies a subset of patients with increased proliferation of polycythemia vera and higher risk of thrombosis.
Copyright© Ferrata Storti Foundation.
Figures


Similar articles
-
Therapeutic options for essential thrombocythemia and polycythemia vera.Semin Oncol. 2002 Jun;29(3 Suppl 10):10-5. doi: 10.1053/sonc.2002.33755. Semin Oncol. 2002. PMID: 12096352 Review.
-
Cardiovascular events and intensity of treatment in polycythemia vera.N Engl J Med. 2013 Jan 3;368(1):22-33. doi: 10.1056/NEJMoa1208500. Epub 2012 Dec 8. N Engl J Med. 2013. PMID: 23216616 Clinical Trial.
-
Low-risk polycythemia vera treated with phlebotomies: clinical characteristics, hematologic control and complications in 453 patients from the Spanish Registry of Polycythemia Vera.Ann Hematol. 2022 Oct;101(10):2231-2239. doi: 10.1007/s00277-022-04963-z. Epub 2022 Aug 30. Ann Hematol. 2022. PMID: 36042023 Free PMC article.
-
Diagnosis and Treatment of Polycythemia Vera: A Review.JAMA. 2025 Jan 14;333(2):153-160. doi: 10.1001/jama.2024.20377. JAMA. 2025. PMID: 39556352 Review.
-
Complex karyotype in a polycythemia vera patient with a novel SETD1B/GTF2H3 fusion gene.Am J Hematol. 2014 Apr;89(4):438-42. doi: 10.1002/ajh.23659. Am J Hematol. 2014. PMID: 24382738
Cited by
-
Real-world analysis of main clinical outcomes in patients with polycythemia vera treated with ruxolitinib or best available therapy after developing resistance/intolerance to hydroxyurea.Cancer. 2022 Jul 1;128(13):2441-2448. doi: 10.1002/cncr.34195. Epub 2022 Apr 13. Cancer. 2022. PMID: 35417564 Free PMC article.
-
Hydralazine Associated With Reduced Therapeutic Phlebotomy Frequency in a Nationwide Cohort Study: Real-World Effectiveness for Drug Repurposing.Front Pharmacol. 2022 Apr 1;13:850045. doi: 10.3389/fphar.2022.850045. eCollection 2022. Front Pharmacol. 2022. PMID: 35431926 Free PMC article.
-
Platelets Contribution to Thrombin Generation in Philadelphia-Negative Myeloproliferative Neoplasms: The "Circulating Wound" Model.Int J Mol Sci. 2021 Oct 20;22(21):11343. doi: 10.3390/ijms222111343. Int J Mol Sci. 2021. PMID: 34768772 Free PMC article. Review.
-
The impact of phlebotomy and hydroxyurea on survival and risk of thrombosis among older patients with polycythemia vera.Blood Adv. 2018 Oct 23;2(20):2681-2690. doi: 10.1182/bloodadvances.2018021436. Blood Adv. 2018. PMID: 30333100 Free PMC article.
-
Myeloproliferative Neoplasms: Contemporary Review and Molecular Landscape.Int J Mol Sci. 2023 Dec 12;24(24):17383. doi: 10.3390/ijms242417383. Int J Mol Sci. 2023. PMID: 38139212 Free PMC article. Review.
References
-
- Gruppo Italiano Studio Policitemia. Polycythemia vera: the natural history of 1213 patients followed for 20 years. Gruppo Italiano Studio Policitemia. Ann Intern Med. 1995;123(9):656–664. - PubMed
-
- Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–2232. - PubMed
-
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005; 434(7037): 1144–1148. - PubMed
-
- Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous hematocrit in primary proliferative polycythaemia. Lancet. 1978;2(8102):1219–1222. - PubMed
-
- Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446–2452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

