Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma
- PMID: 27686492
- PMCID: PMC5041531
- DOI: 10.1186/s13045-016-0334-6
Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma
Abstract
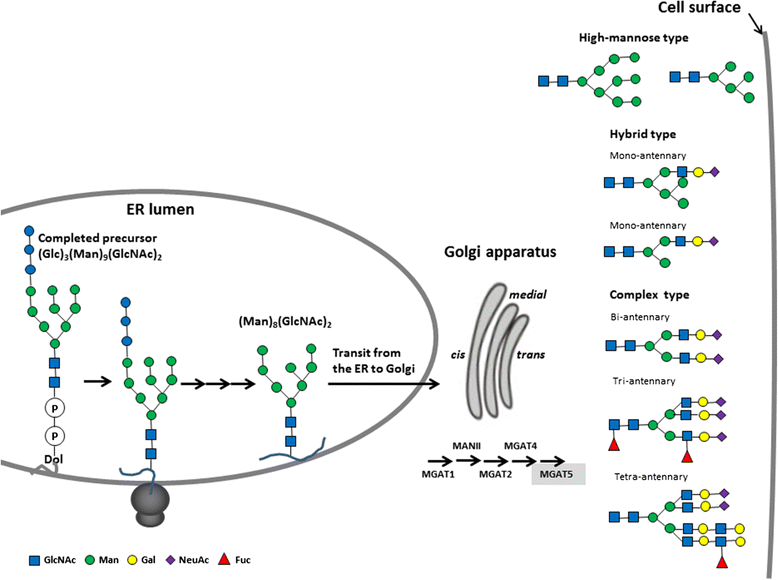
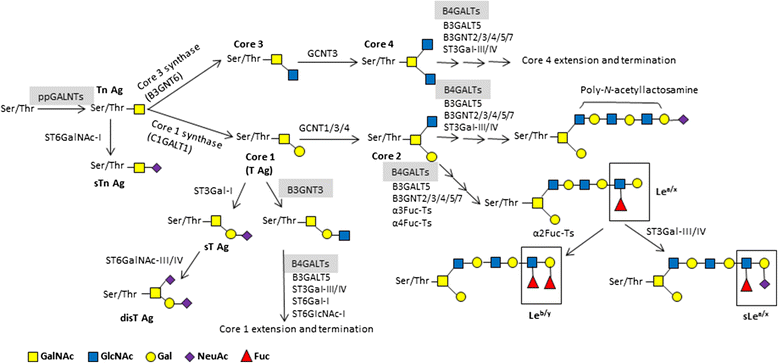
Glycosylation is the most complex post-translational modification of proteins. Altered glycans on the tumor- and host-cell surface and in the tumor microenvironment have been identified to mediate critical events in cancer pathogenesis and progression. Tumor-associated glycan changes comprise increased branching of N-glycans, higher density of O-glycans, generation of truncated versions of normal counterparts, and generation of unusual forms of terminal structures arising from sialylation and fucosylation. The functional role of tumor-associated glycans (Tn, sTn, T, and sLea/x) is dependent on the interaction with lectins. Lectins are expressed on the surface of immune cells and endothelial cells or exist as extracellular matrix proteins and soluble adhesion molecules. Expression of tumor-associated glycans is involved in the dysregulation of glycogenes, which mainly comprise glycosyltransferases and glycosidases. Furthermore, genetic and epigenetic mechanisms on many glycogenes are associated with malignant transformation. With better understanding of all aspects of cancer-cell glycomics, many tumor-associated glycans have been utilized for diagnostic, prognostic, and therapeutic purposes. Glycan-based therapeutics has been applied to cancers from breast, lung, gastrointestinal system, melanomas, and lymphomas but rarely to neuroblastomas (NBs). The success of anti-disialoganglioside (GD2, a glycolipid antigen) antibodies sheds light on glycan-based therapies for NB and also suggests the possibility of protein glycosylation-based therapies for NB. This review summarizes our understanding of cancer glycobiology with a focus of how protein glycosylation and associated glycosyltransferases affect cellular behaviors and treatment outcome of various cancers, especially NB. Finally, we highlight potential applications of glycosylation in drug and cancer vaccine development for NB.
Keywords: Cancer; Glycan-based therapeutics; Glycosyltransferase; Lectin; Neuroblastoma; Protein glycosylation; Treatment.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

