Neuroinflammatory component of gray matter pathology in multiple sclerosis
- PMID: 27686563
- PMCID: PMC5115951
- DOI: 10.1002/ana.24791
Neuroinflammatory component of gray matter pathology in multiple sclerosis
Abstract
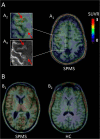
Objective: In multiple sclerosis (MS), using simultaneous magnetic resonance-positron emission tomography (MR-PET) imaging with 11 C-PBR28, we quantified expression of the 18kDa translocator protein (TSPO), a marker of activated microglia/macrophages, in cortex, cortical lesions, deep gray matter (GM), white matter (WM) lesions, and normal-appearing WM (NAWM) to investigate the in vivo pathological and clinical relevance of neuroinflammation.
Methods: Fifteen secondary-progressive MS (SPMS) patients, 12 relapsing-remitting MS (RRMS) patients, and 14 matched healthy controls underwent 11 C-PBR28 MR-PET. MS subjects underwent 7T T2*-weighted imaging for cortical lesion segmentation, and neurological and cognitive evaluation. 11 C-PBR28 binding was measured using normalized 60- to 90-minute standardized uptake values and volume of distribution ratios.
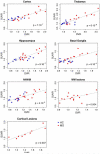
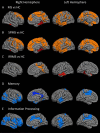
Results: Relative to controls, MS subjects exhibited abnormally high 11 C-PBR28 binding across the brain, the greatest increases being in cortex and cortical lesions, thalamus, hippocampus, and NAWM. MS WM lesions showed relatively modest TSPO increases. With the exception of cortical lesions, where TSPO expression was similar, 11 C-PBR28 uptake across the brain was greater in SPMS than in RRMS. In MS, increased 11 C-PBR28 binding in cortex, deep GM, and NAWM correlated with neurological disability and impaired cognitive performance; cortical thinning correlated with increased thalamic TSPO levels.
Interpretation: In MS, neuroinflammation is present in the cortex, cortical lesions, deep GM, and NAWM, is closely linked to poor clinical outcome, and is at least partly linked to neurodegeneration. Distinct inflammatory-mediated factors may underlie accumulation of cortical and WM lesions. Quantification of TSPO levels in MS could prove to be a sensitive tool for evaluating in vivo the inflammatory component of GM pathology, particularly in cortical lesions. Ann Neurol 2016;80:776-790.
© 2016 American Neurological Association.
Figures


Comment in
-
[11 C]PBR-28 positron emission tomography in multiple sclerosis: Neuroinflammation or otherwise?Ann Neurol. 2017 Feb;81(2):323-324. doi: 10.1002/ana.24853. Epub 2017 Jan 23. Ann Neurol. 2017. PMID: 28019656 No abstract available.
-
Reply.Ann Neurol. 2017 Feb;81(2):324-325. doi: 10.1002/ana.24865. Epub 2017 Jan 23. Ann Neurol. 2017. PMID: 28032651 No abstract available.
References
-
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001 Sep;50(3):389–400. - PubMed
-
- Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003 Aug;9(4):323–31. - PubMed
-
- Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007 Apr;130(Pt 4):1089–104. - PubMed
-
- Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011 Sep;134(Pt 9):2755–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

