PLGA nanoparticle encapsulation reduces toxicity while retaining the therapeutic efficacy of EtNBS-PDT in vitro
- PMID: 27686626
- PMCID: PMC5043181
- DOI: 10.1038/srep33234
PLGA nanoparticle encapsulation reduces toxicity while retaining the therapeutic efficacy of EtNBS-PDT in vitro
Abstract
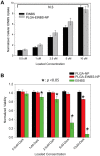
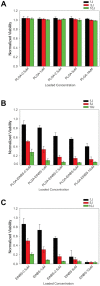
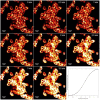
Photodynamic therapy regimens, which use light-activated molecules known as photosensitizers, are highly selective against many malignancies and can bypass certain challenging therapeutic resistance mechanisms. Photosensitizers such as the small cationic molecule EtNBS (5-ethylamino-9-diethyl-aminobenzo[a]phenothiazinium chloride) have proven potent against cancer cells that reside within acidic and hypoxic tumour microenvironments. At higher doses, however, these photosensitizers induce "dark toxicity" through light-independent mechanisms. In this study, we evaluated the use of nanoparticle encapsulation to overcome this limitation. Interestingly, encapsulation of the compound within poly(lactic-co-glycolic acid) (PLGA) nanoparticles (PLGA-EtNBS) was found to significantly reduce EtNBS dark toxicity while completely retaining the molecule's cytotoxicity in both normoxic and hypoxic conditions. This dual effect can be attributed to the mechanism of release: EtNBS remains encapsulated until external light irradiation, which stimulates an oxygen-independent, radical-mediated process that degrades the PLGA nanoparticles and releases the molecule. As these PLGA-encapsulated EtNBS nanoparticles are capable of penetrating deeply into the hypoxic and acidic cores of 3D spheroid cultures, they may enable the safe and efficacious treatment of otherwise unresponsive tumour regions.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

