Memory: Organization and Control
- PMID: 27687117
- PMCID: PMC5499681
- DOI: 10.1146/annurev-psych-010416-044131
Memory: Organization and Control
Abstract
A major goal of memory research is to understand how cognitive processes in memory are supported at the level of brain systems and network representations. Especially promising in this direction are new findings in humans and animals that converge in indicating a key role for the hippocampus in the systematic organization of memories. New findings also indicate that the prefrontal cortex may play an equally important role in the active control of memory organization during both encoding and retrieval. Observations about the dialog between the hippocampus and prefrontal cortex provide new insights into the operation of the larger brain system that serves memory.
Keywords: cognitive control; hippocampus; memory; prefrontal cortex.
Figures
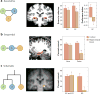



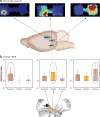
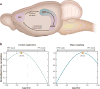
References
-
- Anderson MC, Weaver C. Inhibitory control over action and memory. In: Squire L, editor. Encyclopedia of Neuroscience. Amsterdam, Neth.: Elsevier; 2009. pp. 153–63.
-
- Bartlett FC. Remembering. London: Cambridge Univ. Press; 1932.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

