Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB
- PMID: 27687129
- PMCID: PMC5427517
- DOI: 10.1038/ncomms12976
Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB
Erratum in
-
Corrigendum: Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB.Nat Commun. 2016 Nov 23;7:13768. doi: 10.1038/ncomms13768. Nat Commun. 2016. PMID: 27876812 Free PMC article. No abstract available.
Abstract
Axonal retrograde transport of signalling endosomes from the nerve terminal to the soma underpins survival. As each signalling endosome carries a quantal amount of activated receptors, we hypothesized that it is the frequency of endosomes reaching the soma that determines the scale of the trophic signal. Here we show that upregulating synaptic activity markedly increased the flux of plasma membrane-derived retrograde endosomes (labelled using cholera toxin subunit-B: CTB) in hippocampal neurons cultured in microfluidic devices, and live Drosophila larval motor neurons. Electron and super-resolution microscopy analyses revealed that the fast-moving sub-diffraction-limited CTB carriers contained the TrkB neurotrophin receptor, transiently activated by synaptic activity in a BDNF-independent manner. Pharmacological and genetic inhibition of TrkB activation selectively prevented the coupling between synaptic activity and the retrograde flux of signalling endosomes. TrkB activity therefore controls the encoding of synaptic activity experienced by nerve terminals, digitalized as the flux of retrogradely transported signalling endosomes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
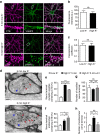
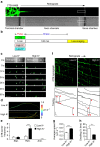

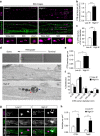
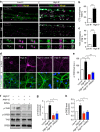
References
-
- Schmieg N., Menendez G., Schiavo G. & Terenzio M. Signalling endosomes in axonal transport: travel updates on the molecular highway. Semin. Cell Dev. Biol. 27, 32–43 (2014). - PubMed
-
- Beattie E. C. et al.. A signaling endosome hypothesis to explain NGF actions: potential implications for neurodegeneration. Cold Spring Harb. Symp. Quant. Biol. 61, 389–406 (1996). - PubMed
-
- Howe C. L. & Mobley W. C. signalling endosome hypothesis: a cellular mechanism for long distance communication. J. Neurobiol. 58, 207–216 (2004). - PubMed
-
- Ye H., Kuruvilla R., Zweifel L. S. & Ginty D. D. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron 39, 57–68 (2003). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

