Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab
- PMID: 27687304
- PMCID: PMC12080753
- DOI: 10.1093/annonc/mdw443
Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab
Abstract
Background: Anti-PD-1 antibodies (anti-PD-1) have clinical activity in a number of malignancies. All clinical trials have excluded patients with significant preexisting autoimmune disorders (ADs) and only one has included patients with immune-related adverse events (irAEs) with ipilimumab. We sought to explore the safety and efficacy of anti-PD-1 in such patients.
Patients and methods: Patients with advanced melanoma and preexisting ADs and/or major immune-related adverse events (irAEs) with ipilimumab (requiring systemic immunosuppression) that were treated with anti-PD-1 between 1 July 2012 and 30 September 2015 were retrospectively identified.
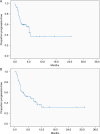
Results: One hundred and nineteen patients from 13 academic tertiary referral centers were treated with anti-PD-1. In patients with preexisting AD (N = 52), the response rate was 33%. 20 (38%) patients had a flare of AD requiring immunosuppression, including 7/13 with rheumatoid arthritis, 3/3 with polymyalgia rheumatica, 2/2 with Sjogren's syndrome, 2/2 with immune thrombocytopaenic purpura and 3/8 with psoriasis. No patients with gastrointestinal (N = 6) or neurological disorders (N = 5) flared. Only 2 (4%) patients discontinued treatment due to flare, but 15 (29%) developed other irAEs and 4 (8%) discontinued treatment. In patients with prior ipilimumab irAEs requiring immunosuppression (N = 67) the response rate was 40%. Two (3%) patients had a recurrence of the same ipilimumab irAEs, but 23 (34%) developed new irAEs (14, 21% grade 3-4) and 8 (12%) discontinued treatment. There were no treatment-related deaths.
Conclusions: In melanoma patients with preexisting ADs or major irAEs with ipilimumab, anti-PD-1 induced relatively frequent immune toxicities, but these were often mild, easily managed and did not necessitate discontinuation of therapy, and a significant proportion of patients achieved clinical responses. The results support that anti-PD-1 can be administered safely and can achieve clinical benefit in patients with preexisting ADs or prior major irAEs with ipilimumab.
Keywords: PD-1; autoimmune disorder; autoimmunity; cancer; immunotherapy; melanoma.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Better use of immune checkpoint inhibition: Treating higher risk patients and examining neurologic toxicity.Ann Oncol. 2017 Feb 1;28(2):204-205. doi: 10.1093/annonc/mdw642. Ann Oncol. 2017. PMID: 27993803 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical