CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion
- PMID: 27687501
- PMCID: PMC5056431
- DOI: 10.1038/ncomms12800
CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion
Abstract
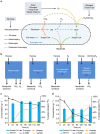
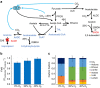
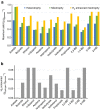
Maximizing the conversion of biogenic carbon feedstocks into chemicals and fuels is essential for fermentation processes as feedstock costs and processing is commonly the greatest operating expense. Unfortunately, for most fermentations, over one-third of sugar carbon is lost to CO2 due to the decarboxylation of pyruvate to acetyl-CoA and limitations in the reducing power of the bio-feedstock. Here we show that anaerobic, non-photosynthetic mixotrophy, defined as the concurrent utilization of organic (for example, sugars) and inorganic (for example, CO2) substrates in a single organism, can overcome these constraints to increase product yields and reduce overall CO2 emissions. As a proof-of-concept, Clostridium ljungdahlii was engineered to produce acetone and achieved a mass yield 138% of the previous theoretical maximum using a high cell density continuous fermentation process. In addition, when enough reductant (that is, H2) is provided, the fermentation emits no CO2. Finally, we show that mixotrophy is a general trait among acetogens.
Conflict of interest statement
White Dog Labs is commercializing a mixotrophy-based fermentation process and has filed a patent application PCT:US2016:019760.
Figures


References
-
- Papoutsakis E. T. Reassessing the progress in the production of advanced biofuels in the current competitive environment and beyond: what are the successes and where progress eludes us and why. Ind. Eng. Chem. Res. 54, 10170–10182 (2015).
-
- Atsumi S., Hanai T. & Liao J. C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451, 86–89 (2008). - PubMed
-
- Bogorad I. W., Lin T.-S. & Liao J. C. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature 502, 693–697 (2013). - PubMed
-
- Fast A. G., Schmidt E. D., Jones S. W. & Tracy B. P. Acetogenic mixotrophy: novel options for yield improvement in biofuels and biochemicals production. Curr. Opin. Biotechnol. 33, 60–72 (2015). - PubMed
-
- Fast A. G. & Papoutsakis E. T. Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals. Curr. Opin. Chem. Eng. 1, 380–395 (2012).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

