Evaluation of the brain-penetrant microtubule-stabilizing agent, dictyostatin, in the PS19 tau transgenic mouse model of tauopathy
- PMID: 27687527
- PMCID: PMC5043530
- DOI: 10.1186/s40478-016-0378-4
Evaluation of the brain-penetrant microtubule-stabilizing agent, dictyostatin, in the PS19 tau transgenic mouse model of tauopathy
Abstract
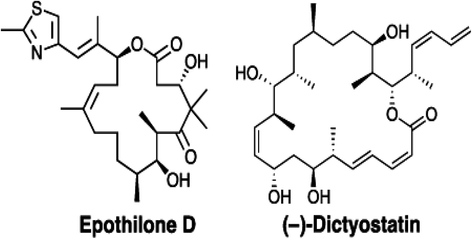
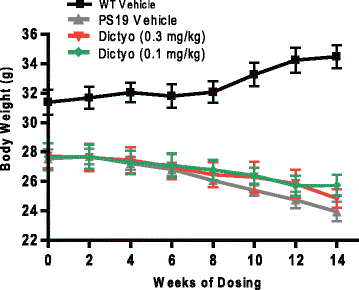
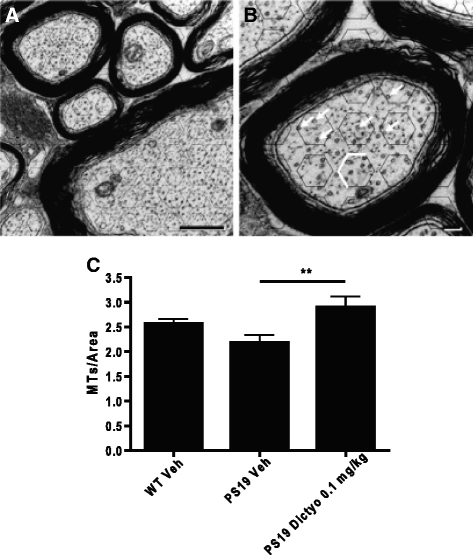
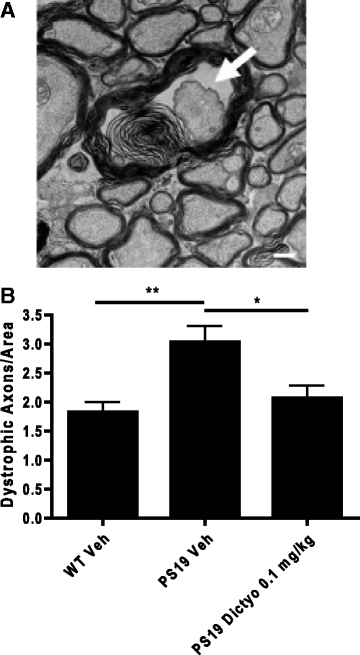
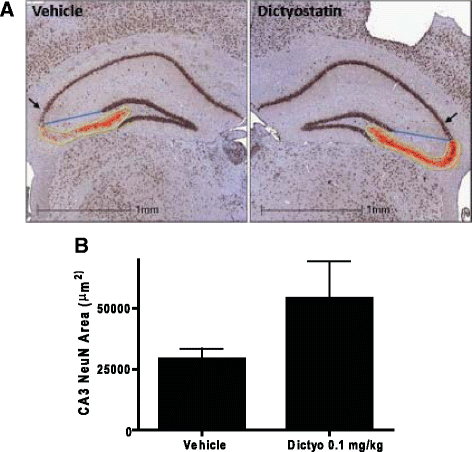
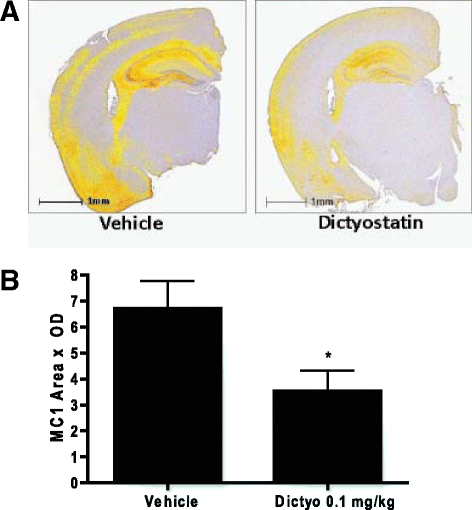
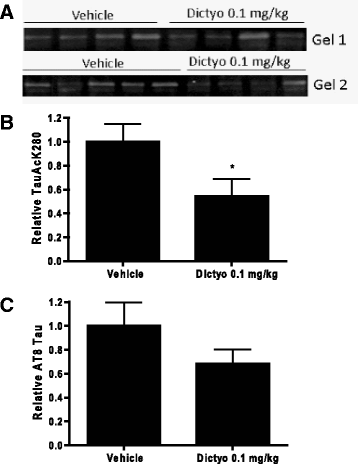
Neurodegenerative disorders referred to as tauopathies, which includes Alzheimer's disease (AD), are characterized by insoluble deposits of the tau protein within neuron cell bodies and dendritic processes in the brain. Tau is normally associated with microtubules (MTs) in axons, where it provides MT stabilization and may modulate axonal transport. However, tau becomes hyperphosphorylated and dissociates from MTs in tauopathies, with evidence of reduced MT stability and defective axonal transport. This has led to the hypothesis that MT-stabilizing drugs may have potential for the treatment of tauopathies. Prior studies demonstrated that the brain-penetrant MT-stabilizing drug, epothilone D, had salutary effects in transgenic (Tg) mouse models of tauopathy, improving MT density and axonal transport, while reducing axonal dystrophy. Moreover, epothilone D enhanced cognitive performance and decreased hippocampal neuron loss, with evidence of reduced tau pathology. To date, epothilone D has been the only non-peptide small molecule MT-stabilizing agent to be evaluated in Tg tau mice. Herein, we demonstrate the efficacy of another small molecule brain-penetrant MT-stabilizing agent, dictyostatin, in the PS19 tau Tg mouse model. Although dictyostatin was poorly tolerated at once-weekly doses of 1 mg/kg or 0.3 mg/kg, likely due to gastrointestinal (GI) complications, a dictyostatin dose of 0.1 mg/kg was better tolerated, such that the majority of 6-month old PS19 mice, which harbor a moderate level of brain tau pathology, completed a 3-month dosing study without evidence of significant body weight loss. Importantly, as previously observed with epothilone D, the dictyostatin-treated PS19 mice displayed improved MT density and reduced axonal dystrophy, with a reduction of tau pathology and a trend toward increased hippocampal neuron survival relative to vehicle-treated PS19 mice. Thus, despite evidence of dose-limiting peripheral side effects, the observed positive brain outcomes in dictyostatin-treated aged PS19 mice reinforces the concept that MT-stabilizing compounds have significant potential for the treatment of tauopathies.
Keywords: Alzheimer’s; Drug; Microtubule; Mouse; Pathology; Tauopathy; Transgenic.
Figures
References
-
- Alonso AD, GrundkeIqbal I, Iqbal K. Abnormally phosphorylated-tau from Alzheimer-disease brain depolymerizes microtubules. Neurobiol Aging. 1994;15:S37.
-
- Barten DM, Fanara P, Andorfer C, Hoque N, Wong PYA, Husted KH, Cadelina GW, Decarr LB, Yang L, Liu V, et al. Hyperdynamic microtubules, cognitive deficits, and pathology Are improved in Tau transgenic mice with Low doses of the microtubule-stabilizing agent BMS-241027. J Neurosci. 2012;32:7137–45. doi: 10.1523/JNEUROSCI.0188-12.2012. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

