E2F coregulates an essential HSF developmental program that is distinct from the heat-shock response
- PMID: 27688402
- PMCID: PMC5066613
- DOI: 10.1101/gad.283317.116
E2F coregulates an essential HSF developmental program that is distinct from the heat-shock response
Abstract
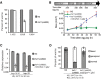
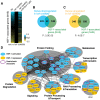
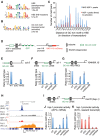
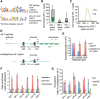
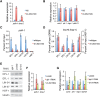
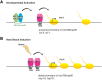
Heat-shock factor (HSF) is the master transcriptional regulator of the heat-shock response (HSR) and is essential for stress resilience. HSF is also required for metazoan development; however, its function and regulation in this process are poorly understood. Here, we characterize the genomic distribution and transcriptional activity of Caenorhabditis elegans HSF-1 during larval development and show that the developmental HSF-1 transcriptional program is distinct from the HSR. HSF-1 developmental activation requires binding of E2F/DP to a GC-rich motif that facilitates HSF-1 binding to a heat-shock element (HSE) that is degenerate from the consensus HSE sequence and adjacent to the E2F-binding site at promoters. In contrast, induction of the HSR is independent of these promoter elements or E2F/DP and instead requires a distinct set of tandem canonical HSEs. Together, E2F and HSF-1 directly regulate a gene network, including a specific subset of chaperones, to promote protein biogenesis and anabolic metabolism, which are essential in development.
Keywords: Caenorhabditis elegans; E2F transcription factor; development; heat-shock factor (HSF); molecular chaperones; stress response; transcription regulation.
© 2016 Li et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

References
-
- Albanese V, Yam AY, Baughman J, Parnot C, Frydman J. 2006. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell 124: 75–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
