Defining the extreme substrate specificity of Euonymus alatus diacylglycerol acetyltransferase, an unusual membrane-bound O-acyltransferase
- PMID: 27688773
- PMCID: PMC5100001
- DOI: 10.1042/BSR20160277
Defining the extreme substrate specificity of Euonymus alatus diacylglycerol acetyltransferase, an unusual membrane-bound O-acyltransferase
Abstract
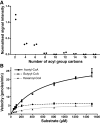
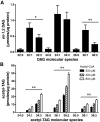
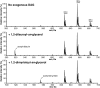
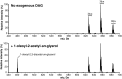
Euonymus alatus diacylglycerol acetyltransferase (EaDAcT) synthesizes the unusually structured 3-acetyl-1,2-diacylglycerols (acetyl-TAG) found in the seeds of a few plant species. A member of the membrane-bound O-acyltransferase (MBOAT) family, EaDAcT transfers the acetyl group from acetyl-CoA to sn-1,2-diacylglycerol (DAG) to produce acetyl-TAG. In vitro assays demonstrated that the enzyme is also able to utilize butyryl-CoA and hexanoyl-CoA as acyl donors, though with much less efficiency compared with acetyl-CoA. Acyl-CoAs longer than eight carbons were not used by EaDAcT. This extreme substrate specificity of EaDAcT distinguishes it from all other MBOATs which typically catalyze the transfer of much longer acyl groups. In vitro selectivity experiments revealed that EaDAcT preferentially acetylated DAG molecules containing more double bonds over those with less. However, the enzyme was also able to acetylate saturated DAG containing medium chain fatty acids, albeit with less efficiency. Interestingly, EaDAcT could only acetylate the free hydroxyl group of sn-1,2-DAG but not the available hydroxyl groups in sn-1,3-DAG or in monoacylglycerols (MAG). Consistent with its similarity to the jojoba wax synthase, EaDAcT could acetylate fatty alcohols in vitro to produce alkyl acetates. Likewise, when coexpressed in yeast with a fatty acyl-CoA reductase capable of producing fatty alcohols, EaDAcT synthesized alkyl acetates although the efficiency of production was low. This improved understanding of EaDAcT specificity confirms that the enzyme preferentially utilizes acetyl-CoA to acetylate sn-1,2-DAGs and will be helpful in engineering the production of acetyl-TAG with improved functionality in transgenic plants.
Keywords: MBOAT; acetyl-TAGs; acetyltransferase; substrate specificity; triacylglycerols.
© 2016 The Author(s).
Figures
References
-
- Durrett T.P., McClosky D.D., Tumaney A.W., Elzinga D.A., Ohlrogge J., Pollard M. A distinct DGAT with sn-3 acetyltransferase activity that synthesizes unusual, reduced-viscosity oils in Euonymus and transgenic seeds. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9464–9469. doi: 10.1073/pnas.1001707107. - DOI - PMC - PubMed
-
- Sidorov R.A., Pchelkin V.P., Zhukov A.V., Vereshchagin A.G., Tsydendambaev V.D. Positional-species composition of triacylglycerols from the arils of mature Euonymus fruits. J. Am. Oil Chem. Soc. 2014;91:2053–2063. doi: 10.1007/s11746-014-2553-8. - DOI
-
- Liu J., Tjellström H., McGlew K., Shaw V., Rice A., Simpson J., Kosma D., Ma W., Yang W., Strawsine M., et al. Field production, purification and analysis of high-oleic acetyl-triacylglycerols from transgenic Camelina sativa. Ind. Crop. Prod. 2015;65:259–268. doi: 10.1016/j.indcrop.2014.11.019. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

