PolyP's many faces
- PMID: 27688781
- PMCID: PMC5043123
- DOI: 10.1182/blood-2016-07-724971
PolyP's many faces
Abstract
In this issue of Blood,
Conflict of interest statement
Conflict-of-interest disclosure: The author declares no competing financial interests.
Figures
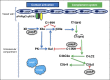
Comment on
-
Polyphosphate is a novel cofactor for regulation of complement by a serpin, C1 inhibitor.Blood. 2016 Sep 29;128(13):1766-76. doi: 10.1182/blood-2016-02-699561. Epub 2016 Jun 23. Blood. 2016. PMID: 27338096 Free PMC article.
References
-
- Engel R, Brain CM, Paget J, Lionikiene AS, Mutch NJ. Single-chain factor XII exhibits activity when complexed to polyphosphate. J Thromb Haemost. 2014;12(9):1513–1522. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

