Review of clinical studies of perampanel in adolescent patients
- PMID: 27688936
- PMCID: PMC5036429
- DOI: 10.1002/brb3.505
Review of clinical studies of perampanel in adolescent patients
Abstract
Aim: To assess the clinical trial and real-world data for adjunctive perampanel in adolescents and develop consensus recommendations to guide the use of perampanel in this population in clinical practice.
Methods: In May 2015, 15 epilepsy experts attended a Consensus Development Meeting to assess the clinical trial data for perampanel, specific to the adolescent age group (12-17 years) and develop consensus treatment recommendations.
Results and discussion: Analysis of the adolescent subgroup data of three pivotal placebo-controlled, double-blind, phase 3 trials investigating perampanel in patients with ongoing focal epileptic seizures despite receiving one to three antiepileptic drugs found that perampanel 4-12 mg was superior to placebo. The tolerability profile of perampanel was generally acceptable. Adolescent patients receiving long-term treatment with perampanel in an open-label extension study maintained improvements in seizure control compared with baseline, with a favorable risk-benefit profile. A phase 2 study showed that perampanel had no clinically important effects on cognitive function, growth, and development.
Conclusion: Perampanel is a welcome addition to the armamentarium of existing antiepileptic drugs as it represents a new approach in the management of epilepsy, with a novel mechanism of action, and the potential to have a considerable impact on the treatment of adolescents with epilepsy.
Keywords: Adolescent; Anticonvulsants; consensus; epilepsy; perampanel; receptors AMPA.
Figures


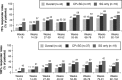

References
-
- Biró, A. , Stephani U., Tarallo T., Bast T., Schlachter K., M. Fleger , et al. 2015. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: first experiences. Neuropediatrics 46:110–116. - PubMed
-
- Centers for Disease Control and Prevention . CDC Growth Charts. Available at http://www.cdc.gov/growthcharts/cdc_charts.htm (accessed February 24, 2016).
-
- French, J. A. , Krauss G. L., Steinhoff B. J., Squillacote D., Yang H., Kumar D., et al. 2013. Evaluation of adjunctive perampanel in patients with refractory partial‐onset seizures: results of randomized global phase III study 305. Epilepsia 54:117–125. - PubMed
-
- Hanada, T. , Hashizume Y., Tokuhara N., Takenaka O., Kohmura N., Ogasawara A., et al. 2011. Perampanel: a novel, orally active, noncompetitive AMPA‐receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 52:1331–1340. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

