Preparation of Thermosensitive Gel for Controlled Release of Levofloxacin and Their Application in the Treatment of Multidrug-Resistant Bacteria
- PMID: 27689094
- PMCID: PMC5027370
- DOI: 10.1155/2016/9702129
Preparation of Thermosensitive Gel for Controlled Release of Levofloxacin and Their Application in the Treatment of Multidrug-Resistant Bacteria
Abstract
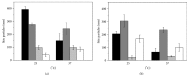
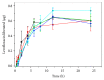
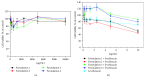
Levofloxacin is a synthetic broad-spectrum antibacterial agent for oral or intravenous administration. Chemically, levofloxacin is the levorotatory isomer (L-isomer) of racemate ofloxacin, a fluoroquinolone antibacterial agent. Quinolone derivatives rapidly and specifically inhibit the synthesis of bacterial DNA. Levofloxacin has in vitro activity against a broad range of aerobic and anaerobic Gram-positive and Gram-negative bacteria. However, formulation of combined poloxamers thermoregulated (as Pluronic® F127) and levofloxacin for use in multiresistant bacterial treatment were poorly described in the current literature. Thus, the aim of the present work is to characterize poloxamers for levofloxacin controlled release and their use in the treatment of multidrug bacterial resistance. Micelles were produced in colloidal dispersions, with a diameter between 5 and 100 nm, which form spontaneously from amphiphilic molecules under certain conditions as concentration and temperature. Encapsulation of levofloxacin into nanospheres showed efficiency and enhancement of antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae when compared with only levofloxacin. Furthermore, all formulations were not cytotoxic for NIH/3T3 cell lineage. In conclusion, poloxamers combined with levofloxacin have shown promising results, better than alone, decreasing the minimal inhibitory concentration of the studied bacterial multiresistance strains. In the future, this new formulation will be used after being tested in animal models in patients with resistant bacterial strains.
Figures
References
-
- Nischal P. M. First global report on antimicrobial resistance released by the WHO. National Medical Journal of India. 2014;27(4):p. 241. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

