Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions
- PMID: 27689804
- PMCID: PMC5070552
- DOI: 10.1021/acs.chemrev.6b00512
Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions
Abstract
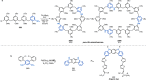
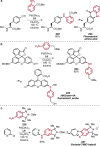
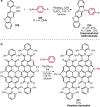
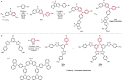
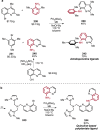
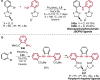
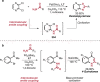
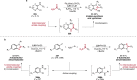
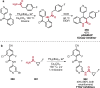
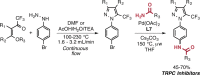
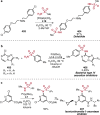
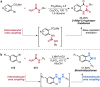
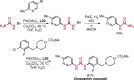
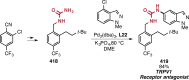
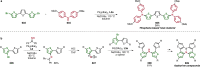
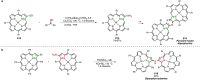
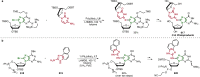
Pd-catalyzed cross-coupling reactions that form C-N bonds have become useful methods to synthesize anilines and aniline derivatives, an important class of compounds throughout chemical research. A key factor in the widespread adoption of these methods has been the continued development of reliable and versatile catalysts that function under operationally simple, user-friendly conditions. This review provides an overview of Pd-catalyzed N-arylation reactions found in both basic and applied chemical research from 2008 to the present. Selected examples of C-N cross-coupling reactions between nine classes of nitrogen-based coupling partners and (pseudo)aryl halides are described for the synthesis of heterocycles, medicinally relevant compounds, natural products, organic materials, and catalysts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


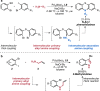
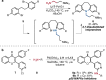

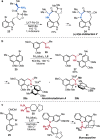
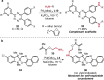

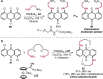
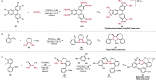
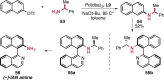

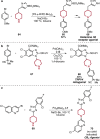
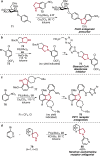
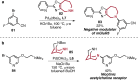

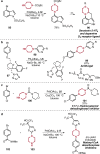
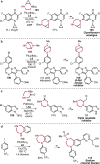
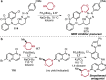
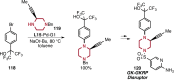


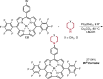
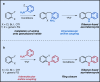

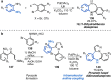

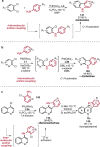
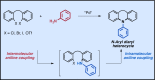
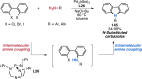
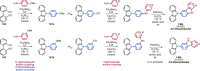
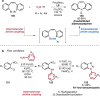
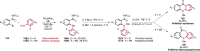
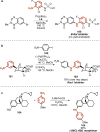

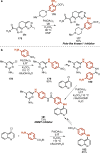



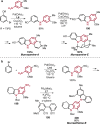
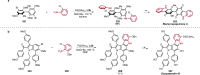
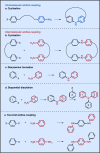

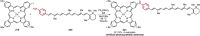

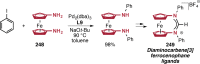
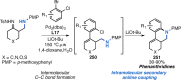


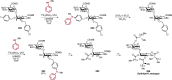

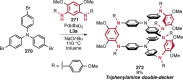
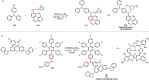
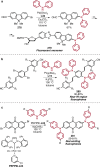
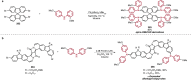
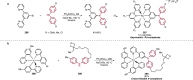
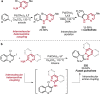
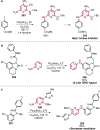




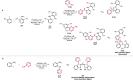
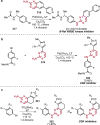
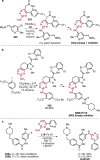
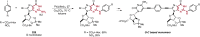
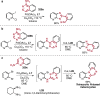
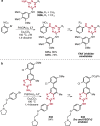
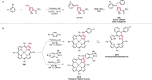
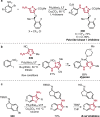

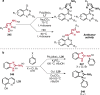
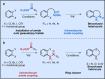
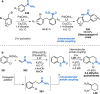
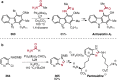
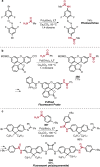

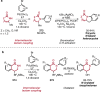
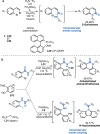
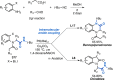
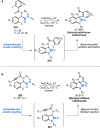
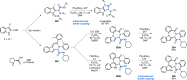


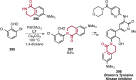



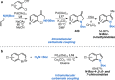
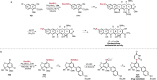
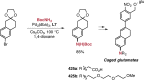
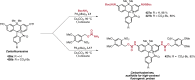
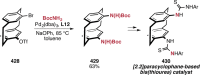
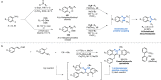
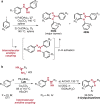


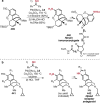
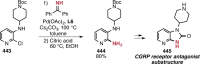
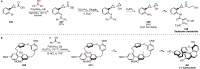

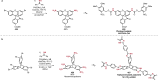
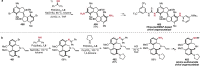
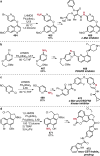


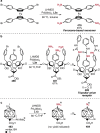

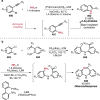
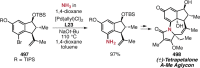



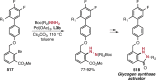
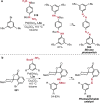
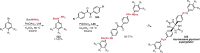
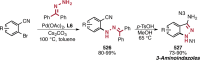
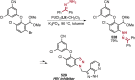


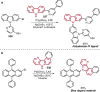


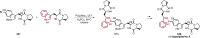

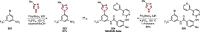
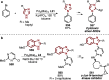
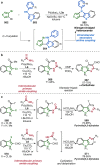

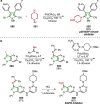

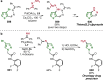


References
-
- Torborg C.; Beller M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351 (18), 3027–3043. 10.1002/adsc.200900587. - DOI
-
- Schlummer B.; Scholz U. Palladium-Catalyzed C-N and C-O Coupling–A Practical Guide from an Industrial Vantage Point. Adv. Synth. Catal. 2004, 346 (13–15), 1599–1626. 10.1002/adsc.200404216. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

