Functional disparities among BCL-2 members in tonsillar and leukemic B-cell subsets assessed by BH3-mimetic profiling
- PMID: 27689871
- PMCID: PMC5260491
- DOI: 10.1038/cdd.2016.105
Functional disparities among BCL-2 members in tonsillar and leukemic B-cell subsets assessed by BH3-mimetic profiling
Abstract
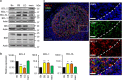
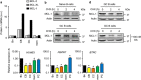
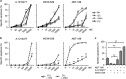
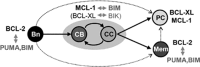
For successful treatment of malignant B-cells it is crucial to understand intrinsic survival requirements in relation to their normal progenitors. Long-lived humoral immunity as well as most B-cell malignancies, originate in the germinal center (GC). Murine GC B-cells depend on pro-survival protein MCL-1, but not BCL-XL. In contrast, naive and memory B-cells depend on BCL-2, but not BCL-XL or MCL-1. For human B-cell subsets, the functional relationships among BCL-2 members are unclear, and also if and how they shift after malignant transformation. We here dissect these aspects in human tonsil and primary leukemia (CLL) cells by single and combined treatment with novel, highly specific BH3-mimetics. We found that MCL-1 expression in GC B-cells is regulated post-translationally and its importance is highlighted by preferential binding to pro-apoptotic BIM. In contrast, BCL-XL is transcriptionally induced and binds solely to weak sensitizer BIK, potentially explaining why BCL-XL is not required for GC B-cell survival. Using novel BH3-mimetics, we found that naive and memory B-cells depend on BCL-2, GC cells predominantly on MCL-1, whereas plasma cells need both BCL-XL and MCL-1 for survival. CLL cells switch from highly sensitive for BCL-2 inhibition to resistant after CD40-stimulation. However, combined inhibition of BCL-2, plus BCL-XL or MCL-1 effectively kills these cells, thus exposing a weakness that may be therapeutically useful. These general principles offer important clues for designing treatment strategies for B-cell malignancies.
Figures


References
-
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014; 15: 49–63. - PubMed
-
- Peperzak V, Vikstrom IB, Tarlinton DM. Through a glass less darkly: apoptosis and the germinal center response to antigen. Immunol Rev 2012; 247: 93–106. - PubMed
-
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–208. - PubMed
-
- Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 2010; 463: 103–107. - PubMed
-
- Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood 1998; 91: 3379–3389. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

