Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections
- PMID: 27689912
- PMCID: PMC5087331
- DOI: 10.1021/acs.jmedchem.6b00948
Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections
Abstract
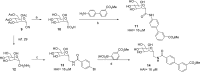
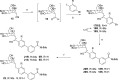
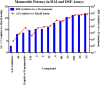
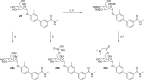
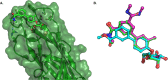
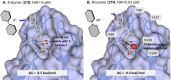
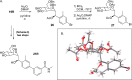
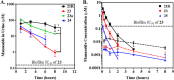
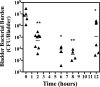
Gram-negative uropathogenic Escherichia coli (UPEC) bacteria are a causative pathogen of urinary tract infections (UTIs). Previously developed antivirulence inhibitors of the type 1 pilus adhesin, FimH, demonstrated oral activity in animal models of UTI but were found to have limited compound exposure due to the metabolic instability of the O-glycosidic bond (O-mannosides). Herein, we disclose that compounds having the O-glycosidic bond replaced with carbon linkages had improved stability and inhibitory activity against FimH. We report on the design, synthesis, and in vivo evaluation of this promising new class of carbon-linked C-mannosides that show improved pharmacokinetic (PK) properties relative to O-mannosides. Interestingly, we found that FimH binding is stereospecifically modulated by hydroxyl substitution on the methylene linker, where the R-hydroxy isomer has a 60-fold increase in potency. This new class of C-mannoside antagonists have significantly increased compound exposure and, as a result, enhanced efficacy in mouse models of acute and chronic UTI.
Conflict of interest statement
The authors declare the following competing financial interest(s): Scott Hultgren and James Janetka are co-founders and stockholders in Fimbrion Therapeutics, Inc.
Figures





References
-
- Boyle E. C.; Finlay B. B. Bacterial pathogenesis: exploiting cellular adherence. Curr. Opin. Cell Biol. 2003, 15, 633–639. 10.1016/S0955-0674(03)00099-1. - DOI - PubMed
- Chagnot C.; Listrat A.; Astruc T.; Desvaux M. Bacterial adhesion to animal tissues: protein determinants for recognition of extracellular matrix components. Cell. Microbiol. 2012, 14, 1687–1696. 10.1111/cmi.12002. - DOI - PubMed
-
- Ofek I.; Beachey E. H.. General concepts and principles of bacterial adherence. In Bacterial Adherence, Receptors and Recognition, Beachey E. H., Ed.; Chapman and Hall: London, 1980; Vol. 6, pp 1–29.
- Ofek I.; Doyle R. J.. Common Themes in Bacterial Adhesion. In Bacterial Adhesion to Cells and Tissues; Chapman and Hall: New York, 1994; pp 513–561.
-
- Lee Y. M.; Almqvist F.; Hultgren S. J. Targeting virulence for antimicrobial chemotherapy. Curr. Opin. Pharmacol. 2003, 3, 513–519. 10.1016/j.coph.2003.04.001. - DOI - PubMed
- Rasko D. A.; Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discovery 2010, 9, 117–128. 10.1038/nrd3013. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

