MicroRNA-375 Functions as a Tumor-Suppressor Gene in Gastric Cancer by Targeting Recepteur d'Origine Nantais
- PMID: 27689991
- PMCID: PMC5085666
- DOI: 10.3390/ijms17101633
MicroRNA-375 Functions as a Tumor-Suppressor Gene in Gastric Cancer by Targeting Recepteur d'Origine Nantais
Abstract
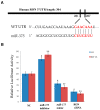
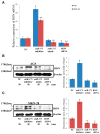
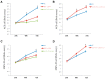
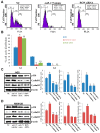
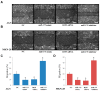
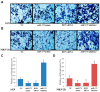
Emerging evidence supports a fundamental role for microRNAs (miRNA) in regulating cancer metastasis. Recently, microRNA-375 (miR-375) was reported to be downregulated in many types of cancers, including gastric cancer. Increase in the expression of Recepteur d'Origine Nantais (RON), a receptor tyrosine kinase, has been reported in tumors. However, the function of miR-375 and RON expression in gastric cancer metastasis has not been sufficiently studied. In silico analysis identified miR-375 binding sites in the 3'-untranslated regions (3'-UTR) of the RON-encoding gene. Expression of miR-375 resulted in reduced activity of a luciferase reporter containing the 3'-UTR fragments of RON-encoding mRNA, confirming that miR-375 directly targets the 3'-UTR of RON mRNA. Moreover, we found that overexpression of miR-375 inhibited mRNA and protein expression of RON, which was accompanied by the suppression of cell proliferation, migration, and invasion in gastric cancer AGS and MKN-28 cells. Ectopic miR-375 expression also induced G1 cell cycle arrest through a decrease in the expression of cyclin D1, cyclin D3, and in the phosphorylation of retinoblastoma (Rb). Knockdown of RON by RNAi, similar to miR-375 overexpression, suppressed tumorigenic properties and induced G1 arrest through a decrease in the expression of cyclin D1, cyclin D3, and in the phosphorylation of Rb. Thus, our study provides evidence that miR-375 acts as a suppressor of metastasis in gastric cancer by targeting RON, and might represent a new potential therapeutic target for gastric cancer.
Keywords: Recepteur d’Origine Nantais; gastric cancer; microRNA-375.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ronsin C., Muscatelli F., Mattei M., Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195–1202. - PubMed
-
- De Bortoli M., Di Renzo M.F., Costantino A., Sismondi P., Comoglio P.M. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927–2933. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

