Recent Advances in Understanding Amino Acid Sensing Mechanisms that Regulate mTORC1
- PMID: 27690010
- PMCID: PMC5085669
- DOI: 10.3390/ijms17101636
Recent Advances in Understanding Amino Acid Sensing Mechanisms that Regulate mTORC1
Abstract
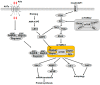
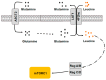
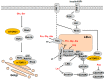
The mammalian target of rapamycin (mTOR) is the central regulator of mammalian cell growth, and is essential for the formation of two structurally and functionally distinct complexes: mTORC1 and mTORC2. mTORC1 can sense multiple cues such as nutrients, energy status, growth factors and hormones to control cell growth and proliferation, angiogenesis, autophagy, and metabolism. As one of the key environmental stimuli, amino acids (AAs), especially leucine, glutamine and arginine, play a crucial role in mTORC1 activation, but where and how AAs are sensed and signal to mTORC1 are not fully understood. Classically, AAs activate mTORC1 by Rag GTPases which recruit mTORC1 to lysosomes, where AA signaling initiates. Plasma membrane transceptor L amino acid transporter 1 (LAT1)-4F2hc has dual transporter-receptor function that can sense extracellular AA availability upstream of mTORC1. The lysosomal AA sensors (PAT1 and SLC38A9) and cytoplasmic AA sensors (LRS, Sestrin2 and CASTOR1) also participate in regulating mTORC1 activation. Importantly, AAs can be sensed by plasma membrane receptors, like G protein-coupled receptor (GPCR) T1R1/T1R3, and regulate mTORC1 without being transported into the cells. Furthermore, AA-dependent mTORC1 activation also initiates within Golgi, which is regulated by Golgi-localized AA transporter PAT4. This review provides an overview of the research progress of the AA sensing mechanisms that regulate mTORC1 activity.
Keywords: amino acids; mTORC1; membrane receptor; membrane transceptor; sensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

