Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites
- PMID: 27690102
- PMCID: PMC5086644
- DOI: 10.3390/toxins8100284
Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites
Abstract
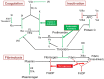
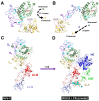
Snake venom metalloproteases, in addition to their contribution to the digestion of the prey, affect various physiological functions by cleaving specific proteins. They exhibit their activities through activation of zymogens of coagulation factors, and precursors of integrins or receptors. Based on their structure-function relationships and mechanism of action, we have defined classification and nomenclature of functional sites of proteases. These metalloproteases are useful as research tools and in diagnosis and treatment of various thrombotic and hemostatic conditions. They also contribute to our understanding of molecular details in the activation of specific factors involved in coagulation, platelet aggregation and matrix biology. This review provides a ready reference for metalloproteases that interfere in blood coagulation, fibrinolysis and platelet aggregation.
Keywords: allosteric sites; anticoagulant; exosites in enzymes; factor X activator; fibrinolytic; platelet aggregation; procoagulant; prothrombin activator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Harvey A.L. Snake Toxins. Pergamon; New York, NY, USA: 1991.
-
- Tu A.T. Reptile Venoms and Toxins. Marcel Decker; New York, NY, USA: 1991.
-
- MEROPS The Peptidase Database. [(accessed on 26 September 2016)]. Available online: http://merops.sanger.ac.uk/
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

